Reviewed by Alex SmithApr 5 2022
When a cat climbs upon a sunflower, what happens? The sunflower is unsteady and will bend fast, causing the cat to tumble to the ground. The sunflower, on the other hand, can operate as a “metastable intermediary step” if the cat just needs a brief boost to grab a bird from there. This is the process by which individual catalyst atoms absorb molecules to chemically alter them.
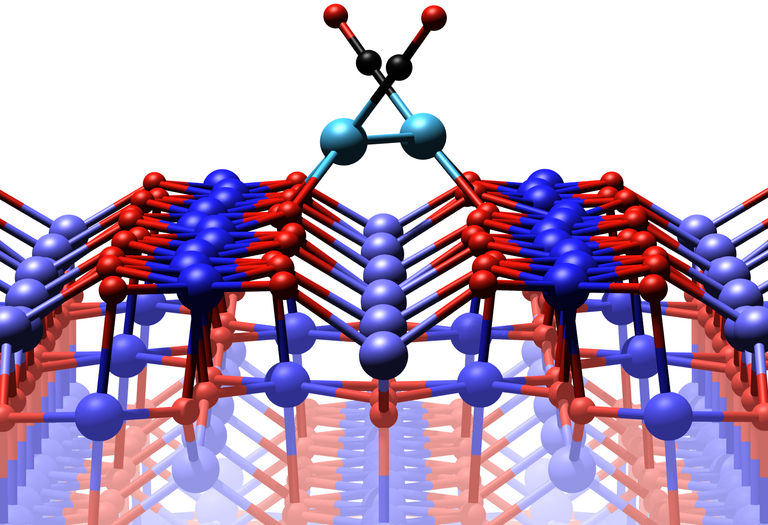 The iron oxide surface with two platinum atoms, each of which is attached to a carbon monoxide molecule. Image Credit: TU Wien.
The iron oxide surface with two platinum atoms, each of which is attached to a carbon monoxide molecule. Image Credit: TU Wien.
The TU Wien surface physics group revealed several years ago that platinum “single-atom” catalysts can oxidize carbon monoxide at temperatures that should not have been achievable based on their theoretical models.
Researchers have now demonstrated that both the catalyst and the material on which it is anchored enter energetically unfavorable “metastable” states for a brief period to allow the reaction to happen uniquely, using atomic-scale microscope pictures and complicated computer simulations. The findings were reported in the journal Science Advances.
Single atoms as catalysts
Prof. Gareth Parkinson’s research group at the Institute of Applied Physics at the TU Wien is researching the tiniest catalysts possible: On an iron oxide surface, individual platinum atoms are deposited. They then come into contact with carbon monoxide gas and convert it to carbon dioxide, just like modern car exhaust does.
This process is technically very important, but exactly what happens when the catalyst is reduced in size to the single atom limit has not been clear until now.
Gareth Parkinson, Professor, Institute of Applied Physics, TU Wien
Prof. Gareth Parkinson stated, “In our research group, we study such processes in several ways: on the one hand, we use a scanning tunneling microscope to produce extremely high-resolution images on which you can study the movement of individual atoms. And on the other hand, we analyze the reaction process with spectroscopy and computer simulations.”
The temperature determines whether the platinum atoms are active as a catalyst. The catalyst is gently and uniformly heated until it reaches the critical temperature, at a point in which carbon monoxide is transformed into carbon dioxide. The threshold at that point is around 550 Kelvin.
However, this did not fit our original computer simulations. According to density functional theory, which is normally used for such calculations, the process could only take place at 800 Kelvin. So we knew: Something important had been overlooked here until now.
Matthias Meier, Study First Author, Institute of Applied Physics, TU Wien
A Metastable State: Short-Lived, But Important
The team collected vast expertise with the same elements in various reactions over several years, and as a consequence, a new image gradually developed. Matthias Meier adds, “With density functional theory, you normally calculate that state of the system that has the lowest energy. That makes sense, because that is the state that the system most often assumes. But in our case, there is a second state that plays a central role: A so-called metastable state.”
Both the platinum atoms and the surface of the iron oxide may transition between distinct quantum physical states. The ground state, which has the least amount of energy, is the most stable.
When a system enters the metastable state, it will eventually return to the ground state within a short period, much like a cat attempting to climb an unstable climbing pole. However, in the catalytic conversion of carbon monoxide, a brief period in the metastable state is sufficient: just as a little period in an unstable climbing condition may be sufficient for a cat to capture a bird with its paw, the catalyst can transform carbon monoxide in the metastable state.
Two platinum atoms join together to form a dimer when carbon monoxide is initially added. The dimer can shift to a less advantageous location where the surface oxygen atoms are less weakly bonded when the temperature is high enough.
The iron oxide modifies its atomic structure precisely at this time in the metastable state, releasing the oxygen atom that the carbon oxide needs to produce carbon dioxide, which immediately flies away, completing the catalytic process. “If we include these previously unaccounted for short-term states in our computer simulation, we get exactly the result that was also measured in the experiment,” explains Matthias Meier.
Our research result shows that in surface physics you often need a lot of experience. If we hadn't studied very different chemical processes over the years, we probably never would have solved this puzzle.
Gareth Parkinson, Professor, Institute of Applied Physics, TU Wien
Artificial intelligence has recently been employed to examine quantum chemical processes with tremendous success — but Parkinson believes that it would not have worked in this situation. After all, researchers will undoubtedly need humans to come up with creative solutions that go beyond what was previously thought possible.
Journal Reference:
Meier, M., et al. (2022) CO oxidation by Pt2/Fe3O4: Metastable dimer and support configurations facilitate lattice oxygen extraction. Science Advances. doi.org/10.1126/sciadv.abn4580.