Ions are typically thought of as single atoms that have gained or lost electrons, but complete molecules can also become ions.
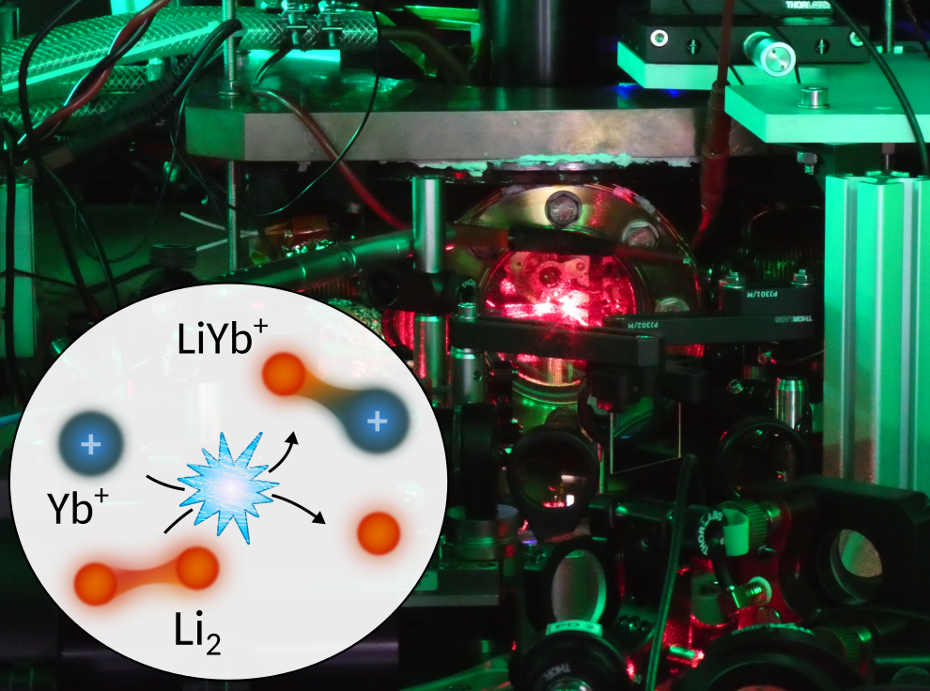 Drawing of the studied collision between a Yb ion and a Li2 Feshbach molecule, resulting in a LiYb+ molecular ion. The background shows a view of the hybrid ion-atom experiment at the University of Amsterdam. Image Credit: University of Amsterdam
Drawing of the studied collision between a Yb ion and a Li2 Feshbach molecule, resulting in a LiYb+ molecular ion. The background shows a view of the hybrid ion-atom experiment at the University of Amsterdam. Image Credit: University of Amsterdam
Scientists from the University of Amsterdam, QuSoft and Stony Brook University demonstrate that cold molecular ions can be formed using a new approach and that they are a beneficial tool for identifying small quantities of other, regular molecules. The study was published as an Editor’s Suggestion in Physical Review Letters.
Trapped Ions
An ion is an atom or molecule with an excess or deficiency of electrons. Ions, as charged particles, can be “trapped” by electromagnetic fields, allowing them to be held in a specific spot.
Ions trapped in a vacuum have the potential for quantum computation. This is because they can be stored for a long time and modern lasers enable physicists to carefully control single ions. These characteristics also make trapped ions ideal candidates for studying chemical reactions, particularly when submerged in a bath of regular atoms or molecules.
Many physics experimental studies benefit from the study of extremely cold particles because cold particles move slower and vibrate less, resulting in very little “noise” in the study.
Ion-molecule experiments have previously been restricted to cold molecules with temperatures around 1 kelvin (one degree above absolute zero), but the hybrid ion-atom experiment at the University of Amsterdam now utilizes molecules with temperatures as low as a few millionths of a kelvin to analyze the world’s coldest ion-molecule collisions.
The molecular ion formed in a chemical reaction where lithium molecules (Li2) and atomic ytterbium ions (Yb+) transform into lithium atoms (Li) and molecular lithium-ytterbium ions (LiYb+), was evaluated by scientists led by Rene Gerritsma from the UvA-Institute of Physics and QuSoft in partnership with Arghavan Safavi-Naini (UvA/QuSoft) and Jesus Pérez-Ríos (Stony Brook University).
Researchers were able to detect very small amounts of molecules using this chemical reaction.
Ultracold Gases
Aside from their several other applications, such as ultraprecise clocks and quantum simulations among several systems, ultracold gases can also be used to produce cold molecules. Using magneto-association, so-called Feshbach dimers can be formed from an ultracold gas — molecules that are as cold as the gas from which they were formed.
IoP physicists Henrik Hirzler, Rianne Lous and Eleanor Trimby witnessed ion-molecule chemical reactions with ultracold molecules for the first time by integrating these molecules with a single trapped ion.
Collisions between a single ion and a Feshbach dimer resulted in the creation of the aforementioned molecular ion, in which one of the molecules’ atoms became stuck to the ion. Looking at the ion’s fluorescence, the creation of the molecular ion can be seen by observing the fluorescence go dark, which is because the molecular ion has different energy levels than the atomic ion.
The existence of the molecular ion was also evaluated by measuring the frequency at which it resonates in the ion trap, varying for heavier molecular particles. Extra measurements showed that every ion-molecule collision did result in the production of a molecular ion.
A Useful Reaction
The scientists then found that their approaches were very sensitive: they could use the reaction Li2 +Yb+ → LiYb+ + Li to distinguish only about 50 molecules in a cloud of 20,000 atoms. Normal imaging methods generally fail when dealing with such minute amounts of molecules.
As a result, the ion could be used as a far superior sensor for molecules. This finding represents an important step toward the ability to explore quantum states of matter using only a single ion as a detector.
Journal Reference:
Hirzler, H., et al. (2022) Observation of Chemical Reactions between a Trapped Ion and Ultracold Feshbach Dimers. Physics Review Letters. doi.org/10.1103/PhysRevLett.128.103401.