Jun 28 2013
Our present understanding of thermodynamics is fundamentally incorrect if applied to small systems and needs to be modified, according to new research from University College London (UCL) and the University of Gdañsk. The work establishes new laws in the rapidly emerging field of quantum thermodynamics.
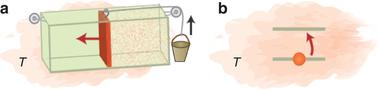 Macroscopic and microscopic work.
Macroscopic and microscopic work.
The findings, published today in Nature Communications, have wide applications in small systems, from nanoscale engines and quantum technologies, to biological motors and systems found in the body.
The laws of thermodynamics govern much of the world around us – they tell us that a hot cup of tea in a cold room will cool down rather than heat up; they tell us that unless we are vigilant, our houses will become untidy rather than spontaneously tidy; they tell us how efficient the best heat engines can be.
The current laws of thermodynamics only apply to large objects, when many particles are involved. The laws of thermodynamics for smaller systems are not well understood but will have implications for the construction of molecular motors and quantum computers, and might even determine how efficient energy extracting processes such as photosynthesis can be.
In this study, researchers used results from quantum information theory to adapt the laws of thermodynamics for small systems, such as microscopic motors, nanoscale devices and quantum technologies.
Small systems behave very differently to large systems composed of many particles. And when systems are very small, then quantum effects come into play. The researchers found a set of laws which determine what happens to such microscopic systems when we heat them up or cool them down. An important consequence of their laws is that there is more fundamental irreversibility in small systems, and this means that microscopic heat engines can not be as efficient as their larger counterparts.
“We see that nature imposes fundamental limitations to extracting energy from microscopic systems and heat engines. A quantum heat engine is not as efficient as a macroscopic one, and will sometimes fail,” said Professor Oppenheim, a Royal Society University Research Fellow at UCL’s Department of Physics and Astronomy and one of the authors of the research. “The limitations are due to both finite size effects, and to quantum effects.”
The researchers investigated the efficiency of microscopic heat engines and found that one of the basic quantities in thermodynamics, the free energy, does not determine what can happen in small systems, and especially in quantum mechanical systems. Instead, several new free energies govern the behaviour of these microscopic systems.
In large systems, if you put pure energy into a system, then you can recover all this energy back to use to power an engine which can perform work (such as lifting a heavy weight). But the researchers found that this was not the case for microscopic systems. If you put work into a quantum system you generally cannot get it all back.
Professor Michal Horodecki of the University of Gdansk, and co-author of the paper, said: “Thermodynamics at the microscopic scale is fundamentally irreversible. This is dramatically different to larger systems where all thermodynamic processes can be made reversible if we change systems slowly enough.”