Reviewed by Alex SmithOct 21 2021
A research study important for the world of science has been carried out by researchers of South Ural State University (SUSU) together with their contemporaries from other research organizations. They have discovered the reasons for the stability of salts and have delivered a thorough description of the results in a renowned journal Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials (Q2).
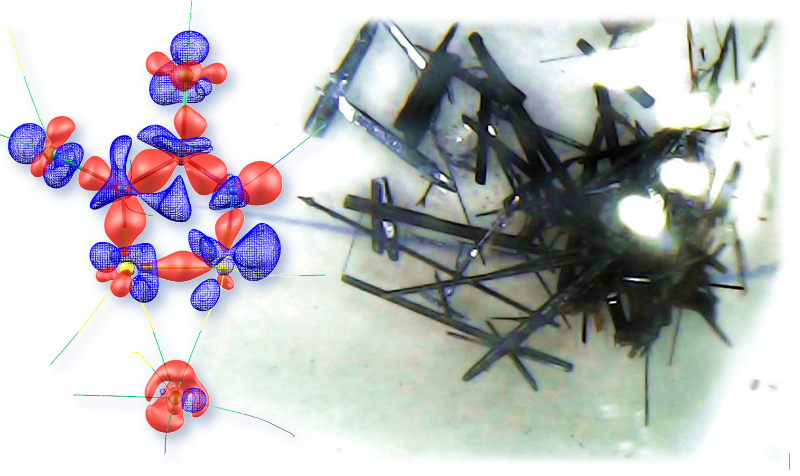
Image Credit: South Ural State University
Research on organic crystals stimulates active interest among researchers, as those are extensively used in numerous fields: from solar cells parts and organic semiconductors to medical chemistry. Experts investigate the different properties of crystals, and among the related subjects, are looking at the specifics of the electron density distribution.
Owing to this information, it becomes likely to predict the chemical and physical, photoelectrical and other properties of materials, which possess the molecular compounds under investigation in their composition.
This research has become one of the fields within the project on “On the way towards new hybrid materials: digital modeling of the structure and properties from the atomic-molecular-level to nanoparticles” being satisfied by the scientists of SUSU along with their contemporaries from research institutes of the Russian Academy of Sciences.
We have become the first in the world to study the experimentally obtained electron density distribution in Appel's salt crystals being the precursor in the synthesis of stable radicals.
Ekaterina Bartashevich, Study Lead and Doctor of Sciences (Chemistry), South Ural State University
Doctor of Sciences (Mathematics and Physics), Professor Vladimir Tsirelson (one of the originators of the scientific domain of quantum crystallography) is formulating a theory, which would enable a quick look at the subatomic level of the structure of multicomponent materials.
To perform the experiment, Candidate of Sciences (Chemistry) Oleg Bolshakov synthesized quality single crystals, guided by the expertise of the study lead, Doctor of Sciences (Chemistry) Oleg Rakitin from N.D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences, who examines the heterocyclic systems based on substituted dithiazoles.
The following step was to capture the X-ray diffraction data in the conditions of uninterrupted cooling. This laborious experiment was handled by Mikhail Miniaev from N.D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences, and Adam Stash from the A.N. Nesmeyanov Institute of Organoelement Compounds.
To develop consistent models and understand the acquired data, the capacities of the SUSU Supercomputer Centre were used; particularly, the work was carried out by Candidate of Sciences (Chemistry) Irina Iushina.
The study produced a vital conclusion: the reason behind the stability of free-radical salts should be pursued at the level of the properties of electron density distribution. They are hidden in the particulars of the formation of chemical bonds, which are of multi-center character.
The researchers will not halt at this juncture. They hope to pursue the research exploration at the level of modeling and at the level of experimental studies of the structure and electronic characteristics of organic crystals.
SUSU is a university of digital transformations, where groundbreaking research is carried out in most of the top-most fields of science and technology development.
In keeping with the strategy of scientific and technological development of the Russian Federation, the university is dedicated to the development of massive scientific interdisciplinary projects in the field of materials science, digital industry, and ecology.
In the Year of Science and Technology, SUSU has become the winner in the competition under the Priority-2030 program. The university serves as a regional project office of the World-class Ural Interregional Research and Education Centre (UIREC).
Journal Reference:
Bartashevich, E., et al. (2021) Bonding features in Appel’s salt from the orbital-free quantum crystallographic perspective. Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials. doi.org/10.1107/S2052520621005928.