The key to resolving the issue of soot pollution could soon be achieved, thanks to research unraveling a hidden molecular dance.
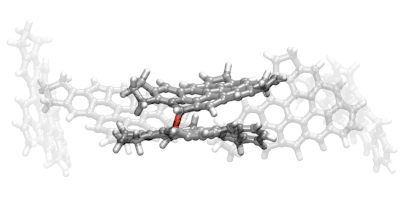 Reactive dimerization of pi-diradicals. Image Credit: Jacob Martin.
Reactive dimerization of pi-diradicals. Image Credit: Jacob Martin.
Soot pollution invades human bodies, resulting in blood clots and cancer and weakening people to respiratory viruses. Also, the glaciers and atmosphere are wrapped by soot, causing global heating and increased ice loss. Unexpectedly, the method of soot particles developing is yet to be discovered but is of serious concern to resolve these worldwide issues.
This has been a long-lasting puzzle because of the extreme environment under which soot tends to develop, the fast speed of the reactions, and the complicated collection of molecules in the flame. All of these features hide the pathway to soot formation.
An international group including researchers from Italy, Switzerland, Singapore, and the United Kingdom employed two different microscopes to unravel the molecules and reactions that occur in a flame.
The first microscope works by the sense of touch and feels the arrangement of atoms in the molecules of soot. Such tactile maps offer a fresh picture of soot’s molecular chicken wire shape. Then, quantum chemistry was employed to reveal that one of the molecules is a reactive diradical. A diradical molecule consists of reactive sites, enabling it to sustain a series of chain reactions.
The second microscope is completely virtual and demonstrates the reaction between the diradicals. Quantum mechanics directed a supercomputer to realistically and virtually collide the molecules and uncover the molecular dance in slow motion.
The simulation has revealed that the separate molecules are fastened by intermolecular forces following their collision. This provides the time for reactive sites to identify each other and make a permanent chemical bond. Even after their bonding is done, they tend to remain reactive, enabling more molecules to “stick” to what is a quickly growing soot particle.
This breakthrough could help resolve the issues faced by earlier attempts to describe soot formation through either a physical condensation or chemical reaction. Indeed, both are needed to describe the quick and high-temperature reactions sufficiently.
According to Jacob Martin, one of the lead authors of the study, “If the concentration of these species is high enough in flames, this pathway could provide an explanation for the rapid formation of soot.”
The project brought together cutting-edge computational modeling and experiments to reveal a completely new reaction pathway which potentially explains how soot is formed. Scientists and engineers have been working on solving this important problem for decades.
Markus Kraft, Study Co-Author, Department of Chemical Engineering and Biotechnology, University of Cambridge
The team plans to focus on these reactive sites to observe if the process of soot formation can be terminated in its tracks. One potential option is the injection of ozone into a flame, which has earlier been identified to remove soot in certain preliminary outcomes in other studies effectively.
This study was financially supported by the National Research Foundation, Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) program.
π-Diradical aromatic soot precursors in flames
Molecular dynamics simulation of reactive dimerization of pi-diradicals. Video Credit: Laura Pascazio.
Journal Reference:
Martin, J. W., et al. (2021) π-Diradical Aromatic Soot Precursors in Flames. Journal of the American Chemical Society. doi.org/10.1021/jacs.1c05030.