May 13 2020
Measurement of the flight times of electrons discharged from a particular atom in a molecule when it is excited with laser light has enabled physicists to quantify the impact of the molecule itself on the kinetics of emission.
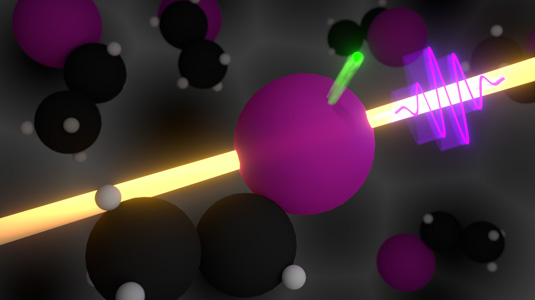 An ultrashort x-ray laser pulse (in violet) removes an inner-shell electron from the iodine atom in ethyl iodide. The experiment times the propagation of the electron with attosecond precision, and measures how much the released electron is decelerated or accelerated by intramolecular forces. Image Credit: Philipp Rosenberger.
An ultrashort x-ray laser pulse (in violet) removes an inner-shell electron from the iodine atom in ethyl iodide. The experiment times the propagation of the electron with attosecond precision, and measures how much the released electron is decelerated or accelerated by intramolecular forces. Image Credit: Philipp Rosenberger.
Photoemission can be described as a process where electrons are emitted in response to excitation by light. It is one of the most basic processes in the microcosm. The kinetic energy of the released electron depends on the concerned atom, and it is governed by the wavelength of the light used.
But what is the time taken for the process? And does it always need the same time, regardless of whether the electron is released from an individual atom or from an atom that forms part of a molecule?
An international research team headed by laser physicists from the Laboratory for Attosecond Physics (LAP) at LMU Munich and the Max Planck Institute of Quantum Optics (MPQ) in Garching has now investigated the impact of the molecule on photoemission time.
When Albert Einstein came up with the theoretical description for photoemission in 1905, it was a major advancement in quantum physics. The details of the process have continued to gain wider attention in the field of science and beyond.
The way in which the motions of an elementary quantum particle (for example, the electron) are influenced within a molecular environment has a considerable impact on scientists’ insights into the process of photoemission and the forces that bind molecules together.
Working closely with researchers from the King Saud University (KSU) in Riyadh (Saudi Arabia), and other international collaborators, the researchers at LAP have now measured the time taken for electrons to be photo-emitted from a particular atom within a molecule (here, it was iodine in ethyl iodide). The quantifies times were in the range of tens of attoseconds, where 1 attosecond is a billionth of a billionth of a second.
The targeted electron was excited by using a range of pulses in the x-ray region. Thanks to machine learning, the precision of the experimental data analysis improved, leading to more accurate comparisons with theoretical predictions.
The comparison of the experimental data with theoretical simulations finally revealed the influence of the molecule on the time that electrons need for the photoemission process.
Matthias Kling, Professor and Head, Ultrafast Imaging and Nanophotonics Group, Laboratory for Attosecond Physics, LMU Munich
It was discovered that the delay caused by the molecular environment started to increase with the decrease in the energy of the light pulses—and thus the initial kinetic energy imparted to the electrons.
The observations are comparable to exploring a landscape. While flying over the landscape, several details on the ground go unnoticed. But at the ground level, each bump can be felt. This holds good in the case of the excited electrons as well.
In case the initial impulse is sufficient only to make them leave the molecule, the retarding effect of the forces that bind the molecule together is higher compared to the value when the “kick” is adequately energetic to release them more promptly.
Our observations indicate that experiments tracing photoemission time permit us to learn about the forces within molecules.
Abdallah Azzeer, Professor and Head, Laboratory for Attosecond Physics, King Saud University
“These studies could improve our understanding of quantum effects in molecules and chemical reactions,” stated Prof. Alexandra Landsman from Ohio State University in the United States, who heads the group that performed a major portion of the theoretical study.
Journal Reference:
Biswas, S., et al. (2020) Probing molecular environment through photoemission delays. Nature Physics. doi.org/10.1038/s41567-020-0887-8.