May 13 2020
The amount of energy needed to make or disintegrate a molecule can now be calculated more accurately than traditional methods using a new machine learning tool. Although the new tool can only deal with simple molecules at present, it opens the door to gain future insights into quantum chemistry.
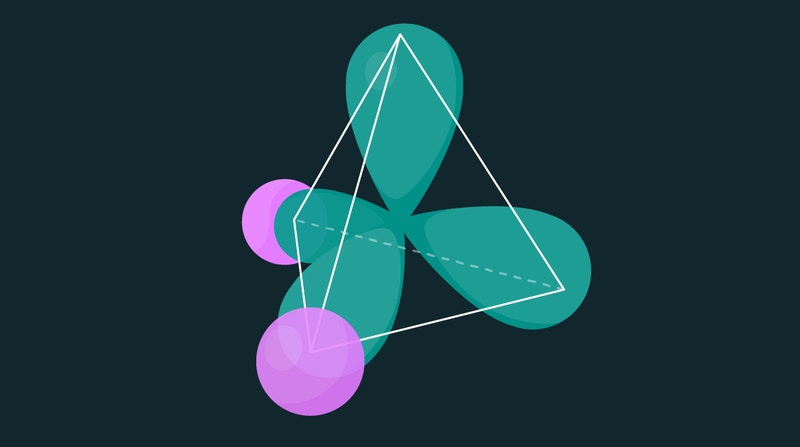 The tetrahedral electronic distribution of a water molecule. The oxygen atom nucleus is at the center of the tetrahedron, and the hydrogen nuclei are in the center of the pink spheres. Image Credit: Simons Foundation.
The tetrahedral electronic distribution of a water molecule. The oxygen atom nucleus is at the center of the tetrahedron, and the hydrogen nuclei are in the center of the pink spheres. Image Credit: Simons Foundation.
Using machine learning to solve the fundamental equations governing quantum chemistry has been an open problem for several years, and there’s a lot of excitement around it right now.
Giuseppe Carleo, Research Scientist, Center for Computational Quantum Physics, Flatiron Institute
Carleo, who is the co-creator of the tool, added that better insights into the formation and degradation of molecules could expose the inner workings of the chemical reactions crucial to life.
Carleo and his colleagues Kenny Choo from the University of Zurich and Antonio Mezzacapo from the IBM Thomas J. Watson Research Center in Yorktown Heights, New York, published their study in Nature Communications on May 12th, 2020.
The tool developed by the researchers predicts the energy required to put together or break apart a molecule, for example, ammonia or water. For this calculation, it is necessary to determine the electronic structure of the molecule, which comprises the collective behavior of the electrons binding the molecule together.
The electronic structure of a molecule is complex to find and requires determining all the possible states the electrons in the molecule could be in, along with the probability of each state.
Electrons interact and entangle quantum mechanically with each other. Therefore, researchers cannot treat them individually. More electrons lead to more entanglements, and thus the problem turns exponentially more challenging.
There are no exact solutions for molecules that are more complex compared to the two electrons found in a pair of hydrogen atoms. Even approximations are not so accurate when more than a few electrons are involved.
One of the difficulties is that the electronic structure of a molecule includes states for an infinite number of orbitals that move further away from the atoms. Moreover, it is not easy to differentiate one electron from another, and the same state cannot be occupied by two electrons. The latter rule is the result of exchange symmetry, which governs the consequences when identical particles change states.
Mezzacapo and the team at IBM Quantum devised a technique for reducing the number of orbitals considered and enforcing exchange symmetry. This technique is based on approaches developed for quantum computing applications and renders the problem more analogous to scenarios in which electrons are restricted to predefined locations, for example, in a rigid lattice.
The problem was made more manageable by the similarity to rigid lattices. Earlier, Carleo trained neural networks to remodel the behavior of electrons restricted to the sites of a lattice.
The researchers could propose solutions to Mezzacapo’s compacted problems by extending those techniques. The neural network developed by the team calculates the probability for each state. This probability can be used to predict the energy of a specific state. The molecule is the most stable in the lowest energy level, also called the equilibrium energy.
Thanks to the innovations of the researchers, the electronic structure of a basic molecule can be calculated quickly and easily. To demonstrate the accuracy of their approaches, the researchers estimated the amount of energy required to break a real-world molecule and its bonds.
The researchers performed calculations for lithium hydride (LiH), dihydrogen (H2), water (H2O), ammonia (NH3), dinitrogen (N2), and diatomic carbon (C2). The researchers’ estimates for all the molecules were found to be highly accurate even in ranges where current methods struggle.
The aim of the researchers is to handle larger and more complex molecules by employing more advanced neural networks. One objective is to tackle chemicals such as those found in the nitrogen cycle, where nitrogen-based molecules are made and broken by biological processes to render them usable for life.
We want this to be a tool that could be used by chemists to process these problems.
Giuseppe Carleo, Research Scientist, Center for Computational Quantum Physics, Flatiron Institute
Carleo, Choo, and Mezzacapo are not the only researchers seeking to use machine learning to handle problems in quantum chemistry. In September 2019, they first presented their study on arXiv.org. In the same month, a research group in Germany and another one at Google’s DeepMind in London reported their studies that involved using machine learning to reconstruct the electronic structure of molecules.
The other two groups made use of a similar method that does not constrain the number of orbitals considered. However, this inclusiveness is more computationally laborious, a disadvantage that will only worsen when more complex molecules are involved.
Using the same computational resources, the method employed by Carleo, Choo, and Mezzacapo produces higher accuracy; however, the simplifications performed to achieve this accuracy could lead to biases.
Overall, it’s a trade-off between bias and accuracy, and it’s unclear which of the two approaches has more potential for the future. Only time will tell us which of these approaches can be scaled up to the challenging open problems in chemistry.
Giuseppe Carleo, Research Scientist, Center for Computational Quantum Physics, Flatiron Institute
Journal Reference:
Choo, K., et al. (2020) Fermionic neural-network states for ab-initio electronic structure. Nature Communications. doi.org/10.1038/s41467-020-15724-9.