Aug 1 2019
Chirality can be a challenging concept for the layperson, with “chemical handedness” seeming a very trivial distinction. However, as the ramifications of the notorious thalidomide disaster prove, understanding chiral materials is a huge concern. The on-going development of chiral separation methods thus remains a core research area.
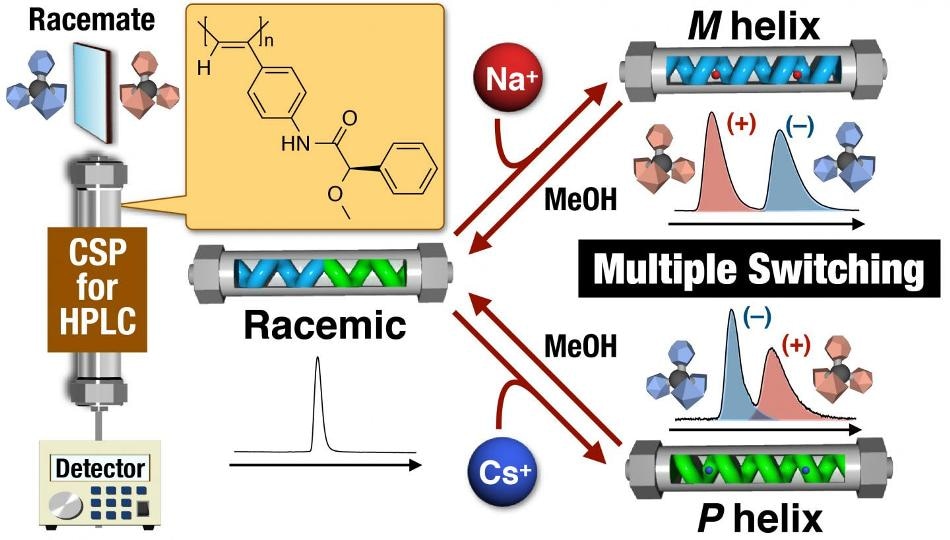 Schematic illustration of three-state switchable chiral stationary phase based on macromolecular helicity modulation in a poly(phenylacetylene) derivative using metal cations in the column. (Image credit: Kanazawa University)
Schematic illustration of three-state switchable chiral stationary phase based on macromolecular helicity modulation in a poly(phenylacetylene) derivative using metal cations in the column. (Image credit: Kanazawa University)
A team comprising scientists from Kanazawa University has reported a three-state switchable chiral stationary phase (CSP) that offers new prospects in chiral separation. Their findings have been published in the Journal of the American Chemical Society.
Chiral high performance liquid chromatography (HPLC) remains the most successful technique for isolating chiral molecules. HPLC requires running samples through a tube—called a column—comprising chiral material (the CSP) that can distinguish between pairs of chiral molecules (enantiomers). However, because of the many enantiomers that cannot be separated using presently available CSPs, investigation in this area is still ongoing.
The scientists describe a CSP based on a helical polymer material having a chiral pendant group that makes the polymer to assume different conformations in reaction to metal ions. When Na+ ions are present, the polymer is made to adopt a left-handed helix so that the ions can relate with the aromatic component of the group.
On the other hand, when Cs+ ions are present, the polymer is pushed into a right-handed helix to enable bonding between the two oxygen atoms and the ions of the group. In the absence of ions, the structure is a neutralized mixture of the two helices.
Our experiments used a CSP based on a poly(phenylacetylene) derivative that can be altered using an achiral external stimulus. This allowed us to control the chirality of the column, and hence the retention of enantiomers, simply by introducing metal salts. The metal ions caused the polymer switch to a particular chiral conformation; however, the effect could be reversed by eluting the column with methanol.
Daisuke Hirose, Study Lead Author, Kanazawa University
The stability of the ion-induced states was shown over four days of nonstop flow through the column, and the separation performance was demonstrated by swapping between the active and deactivated state several times.
As far as we are aware, our system is the first reported example of a CSP that can be switched between three different recognition states using a stimulus that is not chiral. We hope to build on our findings to extend the range of enantiomers that can be separated, which we believe will benefit numerous research areas such as drug discovery.
Katsuhiro Maeda, Study Corresponding Author, Kanazawa University