Mar 18 2019
Researchers at the North Carolina State University have created a microfluidic system for producing perovskite quantum dots over the entire visible light spectrum. The system considerably minimizes manufacturing costs, can be tuned to any color as per requirements, and enables real-time process monitoring to guarantee quality control.
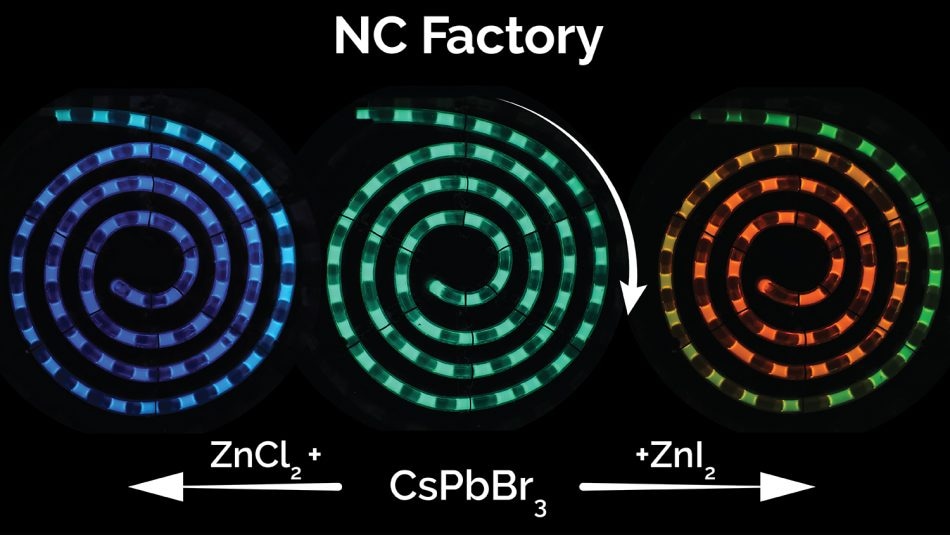 Image credit: Milad Abolhasani, NC State University
Image credit: Milad Abolhasani, NC State University
In the past twenty years, colloidal semiconductor nanocrystals, or quantum dots (QDs), have turned out to be innovative materials for applications as extensive as biological sensing and imaging to solar energy harvesting and LED displays. It would be possible to use the new system to continuously synthesize high-quality QDs for use in such applications.
“We call this system the Nanocrystal (NC) Factory, and it builds on the NanoRobo microfluidic platform that we unveiled in 2017,” stated Milad Abolhasani, an assistant professor of chemical and biomolecular engineering at NC State and corresponding author of a paper on the study.
Not only can we create the QDs in any color using a continuous manufacturing approach, but the NC Factory system is highly modular. This means that, coupled with continuous process monitoring, the system allows modifications to be made as needed to eliminate the batch-to-batch variation that can be a significant problem for conventional QD manufacturing techniques. Additionally, the chemistry we have developed in this work allows the perovskite QD processing to take place at room temperature.
Milad Abolhasani, Assistant Professor of Chemical and Biomolecular Engineering, NC State
The fluorescence color of QDs is caused by the size, the chemical composition, and the manner in which the nanocrystals are processed. The original approach for QD synthesis employed in the NanoRobo system enabled the room-temperature production of green-emitting perovskite QDs, which are developed using cesium lead bromide.
Although NC Factory begins with cesium lead bromide perovskite quantum dots, different halide salts are introduced to exactly tune their fluorescence color over the entire visible light spectrum. The bromine atoms in the green-emitting dots are replaced with either chlorine atoms (to move toward the blue end of the spectrum) or iodine atoms (to move toward red) by the anions in these salts.
Because the NC Factory can precisely control both chemical composition and processing parameters, it can be used to continuously manufacture perovskite quantum dots in any color with the highest quality.
Milad Abolhasani, Assistant Professor of Chemical and Biomolecular Engineering, NC State
There are three “plug and play” modules in the NC Factory system. The scientists created a pre-mixing module to accelerate the mixing of quantum dots and halide salts, to enhance product quality. The system also includes a velocity sensor that enables users to precisely monitor reaction times. Then, the produced QDs are monitored in situ with the help of the NanoRobo process-monitoring module.
“From a scientific standpoint, the NC Factory system allowed us to discover that this halide exchange process takes place in three stages,” stated Abolhasani. “That’s very important for better understanding the reaction mechanism. But the system can also impact practical issues related to quantum dot applications and manufacturing.”
For instance, in the solar power industry, although perovskite quantum dots have gained wider attention for their efficiency, they are still too costly to be used on a large scale. Furthermore, over 60% of that cost is attributed to manufacturing labor.
The NC Factory system would require far less labor to operate continuously. We estimate that the system could cut overall manufacturing costs by at least 50 percent. It should reduce manufacturing costs of QDs for any application and should at least preserve—if not improve—the quality of the quantum dots. We’ve submitted a patent for the system, and are working with industry collaborators to commercialize the technology.
Milad Abolhasani, Assistant Professor of Chemical and Biomolecular Engineering, NC State
The paper titled “Facile Room Temperature Anion Exchange Reactions of Inorganic Perovskite Quantum Dots Enabled by a Modular Microfluidic Platform,” has been published in the Advanced Functional Materials journal. Co-first authors of the paper are Kameel Abdel-Latif and Robert Epps—PhD students in chemical and biomolecular engineering at NC State. The paper was co-authored by Corwin Kerr, an undergraduate student at NC State; Christopher Papa, a PhD student in chemistry at NC State; and Felix Castellano, the Goodnight Innovation Distinguished Chair of Chemistry at NC State.
The study was performed with financial support from a UNC General Assembly Research Opportunities Initiative grant and the Air Force Office of Scientific Research, under grant number FA9550-18-1-0331. The research was also performed with assistance from NC State’s Analytical Instrumentation Facility, supported by the National Science Foundation under grant ECCS-1542015.