Jun 25 2018
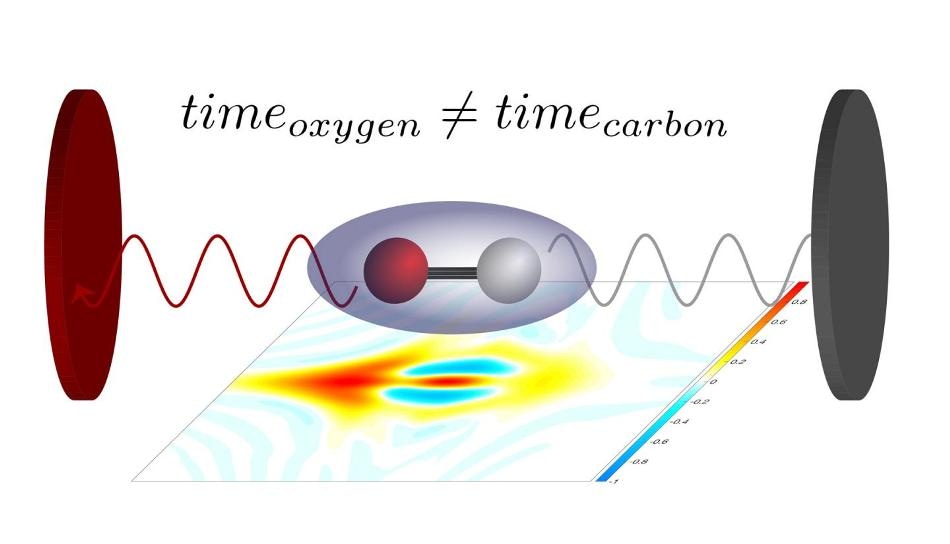 Depending on whether the electron is close to the oxygen or to the carbon atom, the laser pulse will eject it more or less quickly. Image credit: ETH Zurich.
Depending on whether the electron is close to the oxygen or to the carbon atom, the laser pulse will eject it more or less quickly. Image credit: ETH Zurich.
The photoelectric effect is a phenomenon in which an electron from a material is ejected by a photon. Recently, scientists from ETH measured the progression time of this effect in molecules, with the help of attosecond laser pulses. Using the results, it is possible to derive the exact location of a photoionization event.
If a photon with adequate energy hits a material, it can eject an electron from the material. The theoretical interpretation of this phenomenon, called the photoelectric effect, was provided by Albert Einstein in Bern during his “year of wonders” 1905.
That interpretation was a highly significant contribution toward the advancement of quantum mechanics, which was in its development stage, and fetched him the Nobel Prize in Physics in 1921.
Recently, an international group of physicists headed by Ursula Keller from the Institute for Quantum Electronics of the ETH Zurich contributed to this field by adding an innovative dimension to the experimental study of this interesting effect.
The team used attosecond laser pulses to measure a minuscule time difference in the ejection of the electron from a molecule based on the electron’s position inside the molecule.
For quite some time, people have studied the time evolution of the photoelectric effect in atoms, but very little has so far been published on molecules.”
Jannie Vos, PhD Student
That is chiefly because molecules are substantially more complex compared to single atoms. In an atom, the electron moving farthest from the atomic nucleus is basically catapulted out of its orbit.
However, in the case of a molecule, the same electron is shared by two or more nuclei. The location of the electron is dependent on the interplay between the different attractive potentials. Only recently has it been possible to investigate, in detail, the precise way in which the photoelectric effect occurs under these conditions.
To achieve this, Keller and her colleagues used carbon monoxide molecules, which include two atoms—a carbon atom and an oxygen atom. They exposed the molecules to an extreme ultraviolet laser pulse that lasted only for a few attoseconds, where 1 attosecond is equal to a billionth of one-billionth of 1 second.
Due to the high energy of the ultraviolet photons, an electron was ripped out of the molecules, which eventually disintegrated into their constituent atoms. In the process, one of those atoms was transformed into a positively charged ion.
Then, the team used a special instrument to measure the directions in which the electrons and ions flew apart. With the help of a second laser pulse, which functioned similar to a measuring stick, the team determined the exact instant at which the electron was disintegrated from the molecule.
In this way we were able, for the first time, to measure the so-called Stereo Wigner time delay.”
Dr. Laura Cattaneo
The stereo Wigner time delay measured how much earlier or later an electron left the molecule when it is located nearer to the oxygen atom or to the carbon atom during the photoionization effect. Using the extremely short laser pulses, the exact instant, with a precision of within a few attoseconds, can be measured.
With the help of this information, the location of the ionization event inside the molecule can be determined with a precision of within 1/10th of a nanometer. The results of the experiment are in good accordance with theoretical predictions describing the most probable position of an electron when the photoionization occurs.
The next stop for the ETH researchers is to study larger molecules in-depth, starting with the laughing gas N2O. The additional atom in that molecule already renders the theoretical description pretty difficult. However, at the same time, the physicists are confident that new insights can be gained, for instance, into the so-called charge migration inside molecules, which has a vital role in the chemical process.
Ideally, it should even be feasible to use attosecond laser pulses not only to investigation those processes but even to intentionally steer them and hence to regulate chemical reactions in detail. However, at present, such atto-chemistry is still in its evolution.