Apr 18 2018
The main aim of attosecond science is to gain knowledge on the dynamics of quantum-mechanical systems on their natural time scale.
Molecules are one the most intriguing systems to analyze since they have an extremely high degree of complexity, specifically in comparison with atomic systems. To date, the very few attosecond experiments conducted on molecules have offered valuable knowledge related to electron dynamics. In those research works, the researchers presumed the dynamics of the nuclei around which the electrons evolve to be ‘frozen’, provided that nuclei are considerably heavier when compared to electrons and hence move very gradually.
Yet, even in the attosecond time regime, it cannot be justified that electronic motion and nuclear motion are decoupled from each other. Specifically, in the case of molecules formed of light atomic species, the speed of nuclear motion can be equivalent to electron dynamics, leading to a strong coupling between the two.
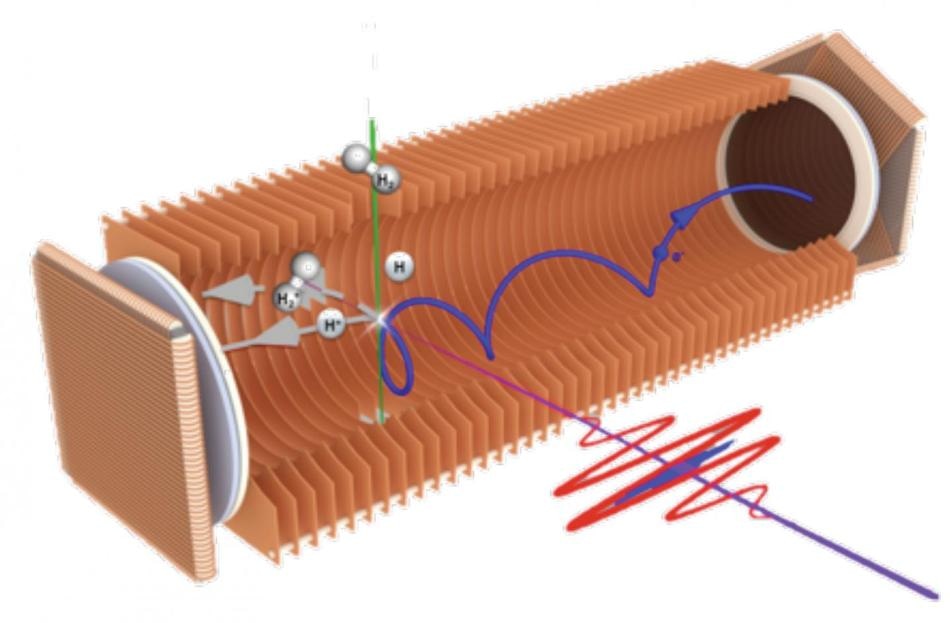 In the AttoCOLTRIMS apparatus, the three-dimensional motion of electrons (blue elliptical arrow) and ions (H2+ and H+, grey arrows) can be detected in coincidence. The combination of an extreme-ultraviolet (XUV, blue) and a long and rather weak infrared (IR, red) pulse in a pump–probe set-up provides the basis for studying the attosecond dynamics of the H2 molecule. (Image credit: Ultrafast Laser Physics group, ETH Zurich)
In the AttoCOLTRIMS apparatus, the three-dimensional motion of electrons (blue elliptical arrow) and ions (H2+ and H+, grey arrows) can be detected in coincidence. The combination of an extreme-ultraviolet (XUV, blue) and a long and rather weak infrared (IR, red) pulse in a pump–probe set-up provides the basis for studying the attosecond dynamics of the H2 molecule. (Image credit: Ultrafast Laser Physics group, ETH Zurich)
At present, a group headed by Dr. Laura Cattaneo and Professor Ursula Keller from the Institute for Quantum Electronics of ETH Zurich has investigated the lightest and smallest of all molecules, H2, and analyzed the results of occurrence of the nuclear and electronic motion on a comparable time scale. The researchers have described in an article published in Nature Physics on April 16, 2018, that in molecules, ionization delays, or the timeframe between the absorption of a photon and the emission of an electron during photoionization, can be more dependent on the kinetic energy of both the photoelectron and the nuclei.
This discovery widens the idea of ionization delays introduced for atomic systems. Differences in ionization delays pertaining to the nuclear kinetic energy can be as large as differences pertaining to the electronic kinetic energy. This indicates that every time light atoms take part in the molecular ionization process, it is not possible to disentangle the outgoing electron wave packet from the nuclear wave packet.
The measurements performed at the attosecond time scale are based on an experimental strategy earlier devised by the Keller team. In the AttoCOLTRIMS device, attosecond metrology is integrated with the imaging method COLTRIMS, wherein the correlated characteristics of the fragments of a molecular reaction can be recorded. This experimental potential was integrated with the almost precise ab initio theory, conducted by collaborators at the Universidad Autónoma de Madrid (Spain), to explain not only the electronic and nuclear motions but also the coupling between them.
The importance of this study is well beyond the simple H2 molecule that was investigated. Hydrogen atoms exist in the majority of the biologically relevant and organic molecules. Gaining in-depth knowledge of the contributions and effects of coupled electron and nuclear dynamics that exist in such systems should hence offer a basic understanding, which will be relevant in different fields of research.