Feb 21 2013
Cornell researchers have resolved a longstanding theoretical debate about the electronic structure of iron-based superconductors by directly observing it at the atomic scale. The work is reported in the Feb. 24 online edition of the journal Nature Physics.
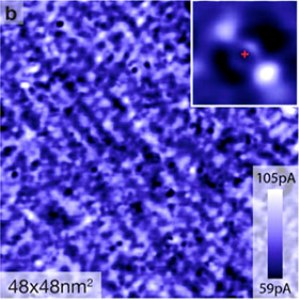 Scanning tunneling microscope image of a 48-nanometer square sample of an iron-based superconductor finds a\n array of dumbbell-shaped "impurities" in the electronic structure, like the sample in the inset, confirming a theoretical prediction. The electronic impurities are associated with cobalt atoms (red cross in the inset). Provided/Davis Lab
Scanning tunneling microscope image of a 48-nanometer square sample of an iron-based superconductor finds a\n array of dumbbell-shaped "impurities" in the electronic structure, like the sample in the inset, confirming a theoretical prediction. The electronic impurities are associated with cobalt atoms (red cross in the inset). Provided/Davis Lab
A team led by J. C. Séamus Davis, the James Gilbert White Distinguished Professor in the Physical Sciences and director of the Center for Emergent Superconductivity at Brookhaven National Laboratory, studied a compound of iron, calcium and arsenic that is "doped" by replacing a few of the iron atoms with cobalt atoms. With around 8 percent doping the material becomes a superconductor. Somehow the cobalt atoms change the environment within this material in a way that allows some electrons to join into "Cooper pairs" that then move without resistance.
Surprisingly, however, when the material is "underdoped" with not quite enough cobalt to create superconductivity, an ordinary electric current moves easily along only one axis of the crystal -- call it "lengthwise" -- but encounters high resistance moving crosswise. This effect increases with the amount of cobalt doping, and may offer a clue to how superconductivity works.
Scanning tunneling microscope (STM) images of the electronic structure of the material in this underdoped state reveal an array of tiny, elongated groups of electrons in an unusual energy state, aligned with the long axis of the crystal, that act as barriers to electrons moving crosswise. It's like having a lot of long, narrow islands in the ocean, all lined up the same way: Boats moving parallel to the islands can slide through between them, but boats going across are blocked and have to detour. The researchers call these structures "impurities" because they differ from the rest of the electronic structure.
"There has been an assumption that the cobalt dopant atoms are neutral," Davis said. "That assumption is wrong. We showed in this project that the dopant atoms do other very surprising things."
Superconductivity was first discovered in metals cooled almost to absolute zero (-273 degrees Celsius), but recently two families of composite materials based on copper and iron have been discovered that superconduct at temperatures as "high" as 135 degrees above absolute zero, offering the tantalizing promise of creating room-temperature superconductivity, with revolutionary consequences for power generation and transport.
In the basement of the Physical Sciences Building on Cornell's Ithaca campus, Davis and his research team have built an STM resting on massive pillars of concrete, so well insulated from vibration that it can scan a tiny probe across a surface in steps smaller than the width of an atom. By measuring current flow between the probe and the surface researchers can determine the energy states of electrons, in effect measuring how much energy it takes to pull an electron loose from its atom. Other measurements can visualize the effects generated when two electrons interact, as when they are scattered by the newly discovered barriers.
This is the first time the impurity scattering associated with dopant atoms, predicted by some theorists but discounted by others, has been observed experimentally, Davis noted. The observations show increasing numbers of the impurities with increased numbers of cobalt atoms, explaining why the one-way resistance increases with cobalt doping, and confirming that the cobalt atoms are responsible, the researchers said. They also said there was strong evidence that magnetism may have something to do with the formation of the impurities -- an intriguing idea, since magnetic fields have been shown to interfere with superconductivity.
The next step, Davis said, is to repeat the experiment with other iron-based superconductors to verify that the phenomenon is universal, and then for materials scientists to try changing the locations of the dopant atoms to see how this changes the electronic structure.
The work was done in collaboration with scientists at several American and European universities, and was supported by the U.S. Department of Energy, the National Science Foundation, the U.K. Engineering and Physical Sciences Research Council and the Netherlands Organization for Scientific Research.