May 11 2016
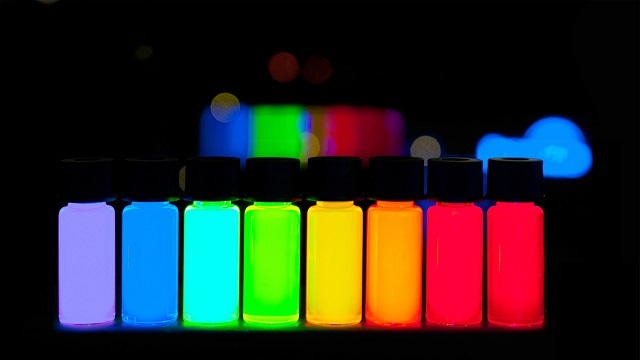
An environmentally friendly, and highly specific, method of biomanufacturing quantum dots (QDs) using a single enzyme has been developed.
QDs are recognized for their electronic and optical properties. These semiconducting nanocrystals produce brilliant and pure colors when induced by ultraviolet light, making them suitable for use in LEDs, solar panels, medical imaging devices and flat screen displays.
However, the chemical manufacturing techniques currently used to produce QDs are expensive and difficult as they involve high pressures and temperatures alongside toxic solvents; hindering their large-scale production and widespread adoption. The novel method, developed by a team at Lehigh University, has addressed these issues, opening the door for a drastically cheaper, quicker and greener QD production method.
The beauty of a biological approach is that it cuts down on the production needs, environmental burden and production time quite a lot.
Prof. Bryan Berger - Lehigh University
in 2015 the team selectively produced cadmium sulfide QDs by modifying the bacterium Stenotophomonas maltophilia using 'directed evolution'. Following this research they discovered that a single enzyme generated by the bacteria is responsible for the production of QDs. This discovery allowed the researchers to completely scrap the cell-based production method. Instead, they produced the same QD-producing enzyme using an engineered strain of E. coli.
We have evolved the enzyme beyond what nature intended
Prof. Bryan Berger - Lehigh University
Specific engineering of the enzymes production allows features of the QD, such as crystal structure and size, to be manipulated allowing the production of uniform QD's which all produce a specific, and uniform, color.
Existing industrial processes require several hours to grow QDs and involve additional purifying and processing steps. Whereas the biosynthetic method produces QDs in the full range of sizes (2-3 nm) in water in a continuous, eco-friendly manner at ambient conditions, without the need for post-processing steps to collect the final water-soluble product.
A state-of-the-art scanning transmission electron microscope (STEM) was required for structural analysis of individual nanoparticles. The instrument fires an ultra-fine electron beam across a field of the nanocrystals. A diffraction image, from the scattering of atoms, is then produced on a fluorescent screen and recorded for further analysis.
Scaling up its successful laboratory results into a manufacturing enterprise producing QDs in a cost-effective and environmentally friendly manner is the next step of the researchers. The cost of production for traditional chemical manufacturing methods is $1,000 to $10,000 per gram. The cost could be reduced by a factor of at least 10 using the biomanufacturing method, and the resulting yields are estimated to be on the order of grams per liter for each batch culture.
McIntosh envisages devising a process that involves self-assembly of individual quantum dots into macrostructures, akin to the way artificial tissues are grown in a lab and a mollusk shell grows naturally out of single inorganic nanoparticles.
If we're able to make more of the material and control how it's structured while maintaining its core functionality, we could potentially get a solar cell to assemble itself with quantum dots.
Prof. Bryan Berger - Lehigh University
The New York Times have published an article on the research titled A Curious Tale of Quantum Dots. The results have also been presented in the Proceedings of the National Academy of Sciences (PNAS) under the title Single Enzyme Biomineralization of Cadmium Sulfide Nanocrystals with Controlled Optical Properties.