May 22 2014
When a supernova – the explosion of a distant star —was discovered last year, astrophysicists, with the help of telescopes around the globe, rushed to observe the fireworks.
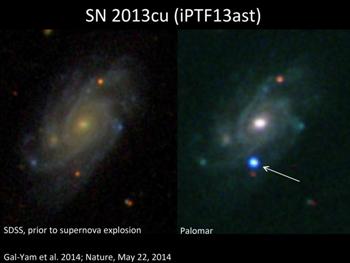 UGC 9379 galaxy imaged in the Sloan digital sky survey before the supernova explosion (left) and by the Palomar Observatory robotic telescope and by the Palomar Observatory robotic telescope afterward (right).
UGC 9379 galaxy imaged in the Sloan digital sky survey before the supernova explosion (left) and by the Palomar Observatory robotic telescope and by the Palomar Observatory robotic telescope afterward (right).
In its dramatic dying flares, this star – a rare type over 10 times the mass of our sun – can tell us something about the life of these fascinating cosmic bodies, as well as helping paint the picture of how all the heavier elements in the universe are formed.
To understand the star that produced the supernova, the researchers identified the mix of elements that was thrown off right before the explosion began. Prof. Avishay Gal-Yam of the Weizmann Institute's Particle Physics and Astrophysics Department explains that the star can be identified by the proportion of such elements as carbon, oxygen and nitrogen detected in the material ejected into space. These elements are created in the nuclear fusion that powers the stars. In our own sun, hydrogen, the lightest atom, fuses to make helium and stops there; but in the massive, hot stars, fusion continues as helium atoms unite to form heavier elements – up to iron.
Scientists believe that such stars are layered like onions: The heaviest elements, for example iron, are located in the core while the lighter ones make up the outermost layers. At the stars' outermost edges are stellar winds that blow the material found there out to space. In stars like the one that exploded the wind is so forceful, it can throw off a mass equal to that of our sun every 10,000 years. At some point in the star's life, the lightweight hydrogen making up its outer layer runs out, and it begins tossing out its helium, oxygen, carbon and nitrogen.
Somewhere under the surface is a layer where hydrogen, helium and the heavier elements all meet. This layer must be high enough to hold hydrogen but still hot enough to produce the extreme temperatures needed for nuclear fusion. Scientists are interested in this layer, as it is the place in which nitrogen is formed. As opposed to carbon, with six protons (three fused helium atoms), or oxygen, with eight (four heliums), nitrogen has an odd number of protons – seven. That means it must be the result of fusion between even and odd atoms, for example, three heliums and a hydrogen. So measuring the quantities of nitrogen could reveal what lies underneath the skin of such stars.
While the wind sweeps away the star's outer layers, its core continues to amass iron until it becomes so heavy it is no longer stable. At that point, the core collapses in a sudden, violent motion, hurling the outer layers off to produce the bright supernova we observe.
Detecting the elements ejected in the stellar wind just before the explosion could only be accomplished within a small window of time – up to a day or so after the terminal blast. This is because intense radiation produced by the explosion shock strips electrons from their atoms. Telescopes equipped with spectrographs aimed at the supernova can pick up the elements' spectra -- light that is emitted when the electrons are reunited with the atoms. But they must make their observations quickly before the quickly-expanding debris from the explosion sweep up the more tenuous remnants of the wind and erase this last trace of the dying star.
The race to observe the spectra of the supernova's wind began with the robotic telescopes at the Palomar observatory in California, a part of the iPTF project led by Prof. Shri Kulkarni of the California Institute of Technology (Caltech). These are programmed to find transient events – sudden changes in the night sky that could be new supernovae – and alert team members about the sightings. Halfway around the world, Dr. Iair Arcavi, then a doctoral student in Gal-Yam's group, received the notification. While researchers in the US slept, he assessed the finding, realized its significance, and contacted Dr. Assaf Horesh, then a postdoctoral fellow at Caltech (who has since joined the Weizmann team). Horesh then conducted spectroscopic observations at the Keck observatory in Hawaii, which is farther west than Palomar and could thus extend the nighttime viewing of the supernova. Acting quickly, he managed to record the emission spectra of the material thrown to the wind a mere 15 hours after the star exploded.
Working backward from the post-blast observations, Gal-Yam, Arcavi, Horesh and their colleagues assessed the recorded spectra and showed that the star that had exploded indeed had a nitrogen-rich wind, similar to those of the so-called Wolf-Rayet stars we know in our galaxy. This is the first time, says Gal-Yam, that this has been done. Now that the team has shown that the combination of efficient global organization and the mobilization of telescopes around the world can work to capture such fleeting events, they hope that further sightings of infant supernova explosions will be possible. Understanding how these stars live and die is important, he says, not just because it gives us a window on the workings of the universe. "All the heavier elements in the universe – those with a mass larger than that of helium – are created in the fusion furnaces of large stars and dispersed through supernova explosions. So many questions – about the origins and the relative abundance of different elements – go back to these processes taking place throughout the cosmos."