May 16 2014
Assembling the puzzles of quantum materials is, in some ways, like dipping a wire hanger into a vat of soapy water, says CIFAR (Canadian Institute for Advanced Research) Fellow Joseph Thywissen (University of Toronto).
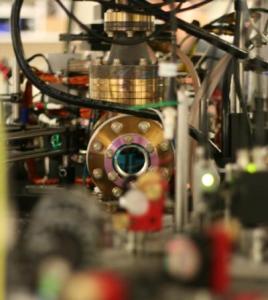 Pictured is a vacuum system that isolates ultracold atoms from room temperature and pressure. Atoms are suspended in this vacuum with laser beams, and manipulated with magnetic fields. A simple photograph taken with an infrared sensitive camera gives information about the atoms. Credit: Denzil Green / Canadian Institute for Advanced Research
Pictured is a vacuum system that isolates ultracold atoms from room temperature and pressure. Atoms are suspended in this vacuum with laser beams, and manipulated with magnetic fields. A simple photograph taken with an infrared sensitive camera gives information about the atoms. Credit: Denzil Green / Canadian Institute for Advanced Research
Long before mathematical equations could explain the shapes and angles in the soap foams, mathematicians conjectured that soap films naturally found the geometry that minimized surface area, thus solving the problem of minimal surfaces. They could be created simply by blowing soap bubbles.
At the University of Toronto's Ultracold Atoms Lab, Thywissen and his team strive to answer what he calls "soap bubble" questions — deep mysteries of the enigmatic quantum materials world that simulations can help us solve. Since the electrons within quantum materials, such as superconductors, zoom far too quickly for careful observation, Thywissen's team uses ultracold gases instead, in this way simulating one quantum system with another, more easily studied, quantum system.
"Simulation gives you the answers but not the theory behind them," says Thywissen.
Thywissen's lab has revealed some of these answers in a new paper about the magnetism and diffusion of atoms in ultracold gases, published in the journal Science. The researchers optically trapped a cloud of gas a billion times colder than air in a very low-pressure vacuum.
They oriented the ultracold atoms, which behave like microscopic magnets, to make them all point in the same direction in space, then manipulated the spins with an effect that's regularly used in hospitals for MRIs, called a spin echo.
Twisting up the direction into a corkscrew pattern and then untwisting it, they measured the strength of interactions between atoms. They observed that at first the atoms did not interact, but one millisecond later they were strongly interacting and correlated.
This rapid change suggested that something was happening to alter the atoms' magnetism as the process unfolded.
"The Pauli Principle forbids identical ultracold atoms from interacting, so we knew something was scrambling the spins at a microscopic level," Thywissen says.
What was happening, the researchers learned next, was diffusion — the same process that takes place when the smell of perfume fills the air of a room, for example.
"If I open a bottle of perfume in the front of the room, it takes a little while for those particles to diffuse to the back of the room," Thywissen says. "They bump into other particles on the way, but eventually get there. You can imagine that the more particles bump into each other, the slower diffusion occurs."
Cranking up interactions to their maximum allowed level, the Toronto team tried to see how slow diffusion could be. They lowered temperature below a millionth of a degree above absolute zero. You might guess that the speed of diffusion would eventually reach zero, but instead the experiment found a lower limit to diffusion.
"Whereas cars on the freeway need to drive below the speed limit, strongly interacting spins need to diffuse above a quantum speed limit," Thywissen says.
Ultracold atoms are just one of a larger family of strongly interacting materials, that also include superconductors and magnetic materials. Thywissen is a member of the CIFAR Quantum Materials program, which is developing an understanding of these materials' novel properties. Cold atoms offer a promising way to explore the mystery of how electrons self-organize to exhibit unusual and valuable properties, such as superconductivity. Quantum materials contain mysteries that have challenged physicists for decades.
"Our measurements imply a diffusivity bound whose mathematical simplicity is exciting: it hints at a universal principle about spin transport, waiting to be uncovered," he says.
Thywissen says CIFAR's support helped make this successful experiment possible.
"CIFAR enabled me to assemble a world-class team."