Mar 13 2014
The research unit that includes Associate Professor Kenji Kojima and Professor Ryosuke Kadono of the Institute of Materials Structure Science, KEK, investigated the magnetic properties of the iridium compound Cu1-xZnxIr2S4 using the muon spin rotation method. This was undertaken in collaboration with Principal Researcher Hiroyuki Suzuki and Unit Director Hideaki Kitazawa of the Advanced Key Technologies Division, NIMS.
The researchers found that the host material with zero zinc concentration, (x = 0) CuIr2S4, is not nonmagnetic as previously believed, but shows novel magnetism below ~ 100 K, and that this magnetism state rapidly disappears when slight amounts of zinc (x ~ 0.01) are substituted.
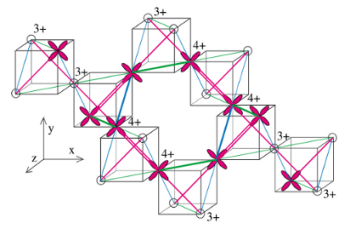 Fig. 1: Framework of iridium (Ir) ions in CuIr2S4. Ir ions are placed at four vertices of each cube, forming tetrahedra. In the insulating state (< 230 K), tetravalent Ir ion octamers form as shown here, and the distance of one out of three types of bonds between Ir (green, blue, and red) is reduced by a few percent. The cross-shaped figures show a schematic of the dxy orbital, which is one of the d orbitals [from Khomskii & Mizokawa, Phys. Rev. Lett. 94, 156402 (2005)]
Fig. 1: Framework of iridium (Ir) ions in CuIr2S4. Ir ions are placed at four vertices of each cube, forming tetrahedra. In the insulating state (< 230 K), tetravalent Ir ion octamers form as shown here, and the distance of one out of three types of bonds between Ir (green, blue, and red) is reduced by a few percent. The cross-shaped figures show a schematic of the dxy orbital, which is one of the d orbitals [from Khomskii & Mizokawa, Phys. Rev. Lett. 94, 156402 (2005)]
Abstract
The iridium compound that was investigated, CuIr2S4, comprises a spinel structure and is metallic at room temperature, but it becomes an insulator below 230 K. Previous structural analysis has showed that, upon becoming an insulator, iridium separates into tetravalent and trivalent ions, each forming octamers. Moreover, one type of bond between tetravalent octamer iridium (green, blue, and red lines in Fig. 1) becomes shorter than the others, and pairs of iridium at both sides of these bonds form four independent pairs. Such structural changes have been confirmed in samples synthesized by the NIMS group through structural analysis using synchrotron X-rays from the KEK Photon Factory.
Research into the magnetic properties of the iridium compound CuIr2S4 using muon spin rotation at J-PARC and TRIUMF (Canada) found an inhomogeneous internal magnetic field below ~ 100 K that is considered to be induced by the magnetic moments of iridium (Fig. 2). Spin glass is one class of magnetic materials that illustrates such magnetism. Previously, CuIr2S4 was believed to have pairs of electron spin when tetrahedra distort in one direction at low temperature, and that this spin singlet state removes magnetic frustration; however, the results presented here reject this hypothesis.
The team also found that substitution of copper with zinc (Cu1-xZnxIr2S4) results in a sudden loss of magnetism in iridium at x ~ 0.01 (1%). Superconductivity is known to appear at x greater than ~ 0.25 in this material, so the relation between magnetism found in this research and superconductivity attracts much interest. As a result, CuIr2S4 is expected to provide a new line of research, different from Sr2IrO4, that further considers the effect of spin-orbit interaction in transition metals.
The discovery in this research is the first experimental evidence concerning the importance of spin-orbit interactions that were previously overlooked in CuIr2S4 and also shows that this material opens up a new area of research with respect to spin-orbit interactions in transition metals. The results were published in the online version of the American academic journal “Physical Review Letters” (February 25, 2014).