Researchers from the University of Chicago, University of California Berkeley, Northwestern University, the University of Colorado Boulder, and Argonne National Laboratory have pioneered a technique using molten salt to grow new types of quantum dots. This technique offers an efficient way to create nanocrystals that were previously inaccessible due to high-temperature requirements. The study was published in the journal Science.
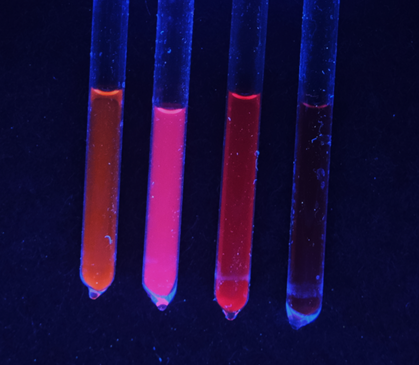 Colloidal solutions of gallium arsenide quantum dots used in lasers, TVs, solar cells, medical devices, and other electronics glow under UV light. These were grown using a groundbreaking technique developed by UChicago’s Talapin Lab, a technique that opens a new world of materials for researchers growing nanocrystals. Image Credit: University of Chicago/Talapin Lab
Colloidal solutions of gallium arsenide quantum dots used in lasers, TVs, solar cells, medical devices, and other electronics glow under UV light. These were grown using a groundbreaking technique developed by UChicago’s Talapin Lab, a technique that opens a new world of materials for researchers growing nanocrystals. Image Credit: University of Chicago/Talapin Lab
Semiconductive nanocrystals known as quantum dots are pushing the boundaries of pure science while powering practical applications in lasers, quantum QLED displays, solar cells, medical devices, and other electronics.
The new technique for growing these microscopic crystals has discovered a more efficient way to create a valuable type of quantum dot, while also unlocking a range of novel chemical materials for future research exploration.
“I am excited to see how researchers across the globe can harness this technique to prepare previously unimaginable nanocrystals,” said First Author Justin Ondry, a Former Postdoctoral Researcher in UChicago’s Talapin Lab.
Researchers achieved these impressive results by substituting molten salt, or superheated sodium chloride, similar to what is sprinkled on baked potatoes, for the organic solvents normally used to create nanocrystals.
Sodium chloride is not a liquid in your mind, but assume you heat it to such a crazy temperature that it becomes a liquid. It looks like liquid. It has a similar viscosity to water. It’s colorless. The only problem was that nobody ever considered these liquids as media for colloidal synthesis.
Dmitri Talapin, Professor, Pritzker School of Molecular Engineering, University of Chicago
Why Salt?
Among the more well-known nanocrystals are quantum dots, both because of their numerous commercial applications and because the team that discovered them was just awarded the 2023 Nobel Prize in Chemistry.
“If there is a material from the world of nano that has had an impact on society in terms of applications, it is the quantum dot,” said UC Berkeley Professor Eran Rabani, Study Co-Author.
However according to Rabani, a large portion of earlier research on quantum dots, including the Nobel work, focused on dots that were created by combining elements from the second and sixth groups of the periodic table. These elements are referred to as "II-VI" (two-six).
There are other intriguing quantum dot materials elsewhere on the periodic table.
This distinct advance of molten salt synthesis that Professor Talapin's group has pioneered for the first time many materials for which previously colloidal synthesis was simply unavailable. Fundamental as well as applied advances can now be made by with many of these newly available materials and at the same time there is now a whole new synthetic frontier available to the community.
Richard D. Schaller, Study Co-Author, University of Chicago
Richard D. Schaller has a joint appointment with Argonne National Laboratory and Northwestern University.
The brightest LEDs, the most potent semiconductor lasers, the fastest electronic devices, and the most efficient solar cells are all made of materials from the third and fifth groups of the periodic table (III-V compounds).
With very few exceptions, they could not be used to generate nanocrystals in solution, but they might form excellent quantum dots. No known organic solvent could withstand the temperatures needed to create these materials.
These previously unavailable materials are now accessible because molten salt can withstand the heat.
The Quantum Age
Zirui Zhou, a Doctoral Student at UChicago and the second author of the new article explained that molten salt's high polarity was one of the reasons researchers synthesizing nanocrystals ignored it.
The negatively and positively charged ions in salt are strongly attracted to one another. Researchers anticipated that because nanocrystals and other small objects have minimal surface charges, the charge would be too weak to resist the pull of salt ions. Before any crystals could develop into a stable substance, they would be smashed.
Or so earlier researchers believed.
Zhou said, “It is a surprising observation. This is very contradictory to what scientists traditionally think about these systems.”
For many members of the research team, the most exciting aspect of the new technique is the opportunity to explore novel materials, which could lead to new building blocks for faster and better quantum and classical computers.
Ondry said, “Many eras in human history are defined by the materials humanity had available—think ‘Bronze Age’ or ‘Iron Age. In this work, we have unlocked the ability to synthesize nearly a dozen new nanocrystal compositions which will enable future technologies.”
Journal Reference:
Ondry, J. C., et al. (2024) Reductive pathways in molten inorganic salts enable colloidal synthesis of III-V semiconductor nanocrystals. Science. doi.org/10.1126/science.ado7088.