In an article recently published in the journal Nanomaterials, researchers investigated the physicochemical properties of cadmium telluride/glutathione (CdTe/glutathione) quantum dots (QDs) synthesized using microwave irradiation for potential application in biomarker and monoclonal antibody testing.
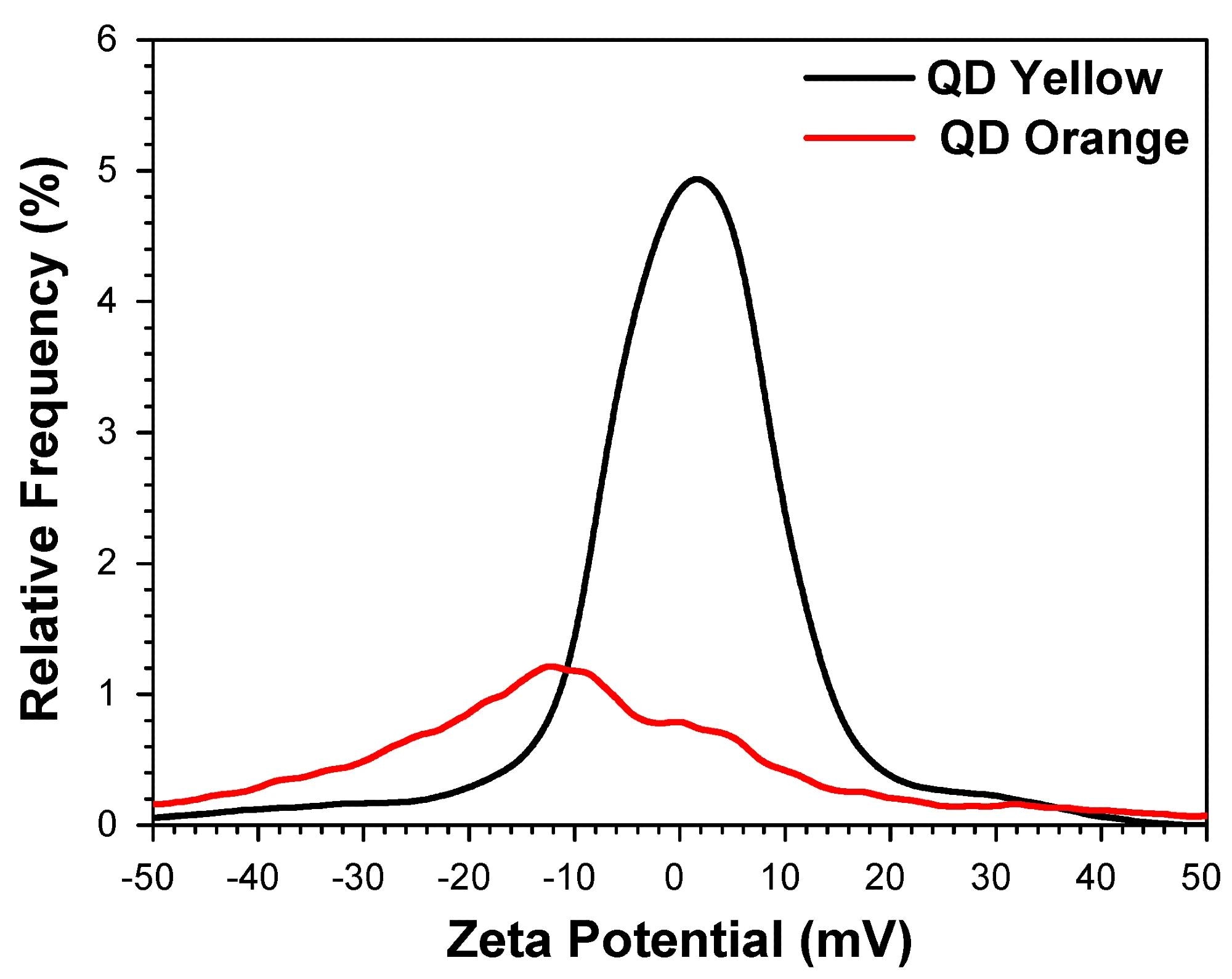 Determination of the average zeta potential. Image Credit: https://www.mdpi.com/2079-4991/14/8/684
Determination of the average zeta potential. Image Credit: https://www.mdpi.com/2079-4991/14/8/684
Importance of QDs
QDs emit wavelengths spanning the infrared to ultraviolet spectra, characterized by high molar extinction coefficients, photostability, and quantum yield. In nanobiotechnology, these materials are crucial for the treatment, diagnosis, and monitoring of biological systems. They enhance drug delivery efficiency to targeted sites within organisms, optimizing signaling and reducing side effects in non-targeted areas.
Among various QDs, those small in size and synthesized in aqueous solutions are preferred as biomarkers due to their biocompatibility and luminescence. These nanoparticles are readily synthesized from many inorganic precursors in aqueous environments, enabling the biotechnology industry to harness their luminescent properties for a range of applications, including use as cellular biomarkers.
Glutathione-stabilized CdTe QDs are particularly noteworthy, offering high brightness, photostability, and tunable emissions. These properties greatly enhance their utility in biological applications, expanding possibilities for protein conjugations and biological imaging to detect polysaccharides.
While the wet solution preparation method is more convenient and cost-effective compared to traditional organometallic methods, it typically results in a lower quantum yield of 1 to 10%. Of all QDs synthesized in aqueous media, to date, only CdTe has demonstrated optical properties comparable to those of their organometallic counterparts.
The Study
In this study, researchers conducted a comprehensive physicochemical characterization—including chemical, optical, and structural analysis—of CdTe QDs stabilized with glutathione. The aim was to optimize the synthesis conditions to assess the suitability of these QDs for use in monoclonal antibody testing and as biomarkers. Specifically, high-quality CdTe-QDs were synthesized in aqueous solutions using glutathione as a stabilizing ligand, with microwave irradiation enhancing the synthesis process.
The synthesis utilized cadmium chloride, trisodium citrate dehydrate, L-glutathione, sodium borohydride, and sodium tellurite as input materials. Post-synthesis, the fluorescence of the CdTe-glutathione QDs was activated using a microwave irradiation-assisted hydrothermal method.
Physicochemical properties were thoroughly evaluated using a suite of techniques: optical absorbance spectroscopy, dynamic light scattering (DLS), electrophoretic light scattering (ELS), static light scattering (SLS), transmission electron microscopy (TEM), X-Ray diffraction (XRD), photoluminescence spectroscopy, inductively coupled plasma–atomic emission spectrometry (ICP-AES), Fourier-transform infrared spectroscopy (FT-IR), and atomic emission spectroscopy (AES).
The stabilization of CdTe QDs was notably enhanced by the glutathione molecule, particularly its thiol group, which has a strong chemical affinity for transition metals like cadmium. This affinity allowed the use of a higher proportion of cadmium precursor relative to the telluride precursor, promoting the formation of a cadmium-rich surface on the nanocrystals. The thiol groups then passivated the valence levels, providing effective stabilization. This surface, characterized by the presence of a mono-thiol compound, is non-cytotoxic and naturally occurs at millimolar concentrations in most organs.
Additionally, glutathione’s amine and carboxyl functional groups play a pivotal role in labeling myeloid cells, as they enable the molecule to bind to specific receptors on the myeloid cell surface, facilitating internalization and initiating an immune response.
Research Findings
The physicochemical analysis of glutathione-stabilized CdTe QDs suggests that these nanocrystals are highly effective for detecting tissues and malignant tumors smaller than 1 cm. An exceptional level of control over emission properties and nanocrystal surface composition was achieved, enhancing their application in monoclonal antibody testing and as biomarkers.
The hydrodynamic sizes of two QD samples, labeled QDYellow and QDOrange based on their emission colors, measured 6.7 nm and 19.4 nm, respectively. Photoluminescence spectroscopy revealed that QDYellow and QDOrange exhibited peak emission intensities at 570 nm and 606 nm, respectively, covering a visible spectrum from yellow to orange.
Infrared spectroscopy identified vibrational modes corresponding to functional groups such as carboxyl, hydroxyl, and amine, facilitating the development of functionalized QDs tailored for biomarker production. Experiments with murine myeloid cells demonstrated that both QDYellow and QDOrange diffused into the cells and interacted with the cell nucleus, confirming their potential in cellular labeling.
Results from the inductively coupled plasma–atomic emission spectrometry confirmed that glutathione-stabilized CdTe QDs internalized into the cells and marked the nuclear, cytoplasmic, and surrounding regions in the cytoplasmic membrane. This was demonstrated through a robust interaction between the deoxyribonucleic acid and ribonucleic acid present in the cells with the QDs, showing their effectiveness as cellular biomarkers.
In summary, glutathione-stabilized CdTe-QDs, synthesized via microwave irradiation, prove effective for both cytoplasmic and nuclear labeling. This capability is pivotal for the precise diagnosis of diseases such as leukemia and lymphoma. Moreover, these QDs have the potential to target specific therapeutic agents or drugs to myeloid cells, thereby enhancing treatment efficacy and reducing adverse effects.
Journal Reference
Ruiz-Robles, M.A.; Solís-Pomar, F.J.; Aguilar, G. T; Mijares, M. M; Arteaga, R. G; Armenteros, O. M; Gutiérrez-Lazos, C.D.; Pérez-Tijerina, E.G.; Cruz, A F. (2024) PhysicoChemical Properties of CdTe/Glutathione Quantum Dots Obtained by Microwave Irradiation for Use in Monoclonal Antibody and Biomarker Testing. Nanomaterials, 14, 684. https://doi.org/10.3390/nano14080684, https://www.mdpi.com/2079-4991/14/8/684
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.