Mass spectrometry has grown and been used to study and produce data for numerous applications at a remarkable rate. Very sensitive, high-performance mass spectrometry is now being utilized using ion trap quantum sensors.

Image Credit: S. Singha/Shutterstock.com
A mass spectrometer can range in size from a penny to a room. The many instruments perform all functions based on the same fundamental principles while having quite different applications.
The precise measurement of a sample's constituent molecules' masses is possible through the use of mass spectrometry MS. MS is an analytical method that uses changes in the ratios of charges to masses (mass/charge; m/z) to separate ionized particles, such as atoms, molecules, and clusters.
In mass spectrometry, there are four steps: ionization, acceleration, deflection, and detection.
Ionization
After the sample has been vaporized, it is put into an ionization chamber and subjected to a stream of electrons produced by a metal coil that has been heated electrically. A positively charged ion is produced when the particle experiences violent collisions that remove one or more of its electrons. Due to the inherent difficulty of extracting a second electron from an already positive ion, the majority of these have a charge of +1.
Different techniques can be used to create ions based on the target analyte under investigation. For instance, electron beam ionization splits molecules into ions by means of an intense electron beam colliding with the target molecule. Ions from an aerosol are created when the eluent from a liquid chromatograph is exposed to electrospray ionization; another technique is laser ablation.
Acceleration
The positively charged ions are repelled by the positively charged ionization chamber and move at a gradually lower voltage towards three negatively charged slits. Since their mass determines how quickly they accelerate, lighter ions travel more quickly than heavier molecules.
Deflection
At this point, a magnetic field deflects the stream of positively charged ions. The mass and charge of the ion determine how much of a deflection there is. The greater the deflection, the lower the mass of the ion. Additionally, ions with charges larger than +1 will deflect more.
Detection
In this last step, a detector uses the mass-to-charge ratio (m/z) to identify the ion beam that is going through the mass analyzer. An electron from the metal jumps onto the ion to neutralize its charge when it strikes the detector. As a result, an electrical current is produced that is proportionate to the ion abundance.
After these four steps are finished, a mass spectrum is produced, which is then analyzed by a computer to display the various m/z values of the ions present and their relative abundance.
Types of Mass Spectrometers
The type of mass spectrometric technology used determines how accurately or efficiently a mass spectrometer can measure the true mass. Here are a few instances:
- Isotope ratio mass spectrometry (IRMS)
- Time of flight mass spectrometry (TOFMS)
- Inductively coupled plasma mass spectrometry (ICP-MS)
- Liquid chromatography-mass spectrometry (LC-MS) and
- Proton transfer reaction-mass spectrometry (PTR-MS)
- Ambient Mass Spectrometry (AMS)
- Gas chromatography-mass spectrometry (GC-MS)
- Quadrupole mass analyzers
Quadrupole Mass Analyzers
Quadrupole mass analyzers are a key technological construct as they have been adapted for quantum sciences.
The four parallel rods with a circular or hyperbolic cross-section make up a quadrupole mass analyzer. As described in the caption of the figure below, these are connected to both direct-current and radio-frequency power supply.
The equations of motion guiding the ions' passage between the rods describe a particular set of oscillations. For certain values of the equations, these oscillations are stable; for other values, they are unstable. The ions will be forced to travel between the rods until they reach the detector by running the quadrupole in a steady area.
The mass spectrum is scanned by varying V0 and U so that the ratio U/V0 remains constant. The quadrupole mass analyzer acts as a mass filter, allowing one mass channel at a time to reach the detector as the mass range is scanned.
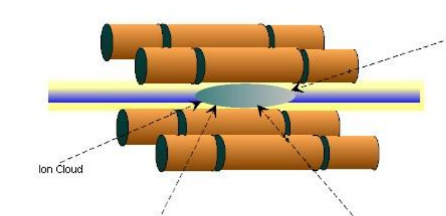
Figure 1: A quadrupole mass analyzer with segmented rods. The middle rods are applied with alternating electric fields while the end rods are held at DC voltage. This configuration constraints charged particle to a localized location in the middle of the four-rod arrangement. Credit: https://doi.org/10.1063/1.4825352
This exact set-up is adapted for ion traps in quantum information science studies.
Ion Trap as a Mass Quantum Spectrometer
While an ion is being trapped and cooled in a trap, a novel gadget known as a quantum sensor has been developed to measure the mass of a single ion with unparalleled sensitivity and accuracy. A single calcium ion serves as the sensor in the quantum sensor; it is laser-cooled to mK temperatures and kept in a second trap that is linked to the trap for the ion under investigation via a common end cap. The image current generated in the common endcap converts the cyclotron motion of the ion under inquiry into axial motion along the magnetic field lines and couples it to the sensor ion.
The sensor ion's axial motion is observed through its fluorescence light, which provides spatial resolution. This allows for the improvement of phonon detection to photon detection. With the use of this apparatus, contemporary constraints in high-precision mass spectrometry can be surmounted.
Outlook
Despite requiring little sample time, mass spectrometry methods yield fast, dependable results that are, most importantly, comparable to those from traditional methods. Improved speed and sophisticated analytical features in software development will raise the accuracy of mass spectrometry results. This in turn can provide valuable insights into quantum chemistry and other challenging topics in the future.
More from AZoQuantum: What to Know About Photonic Quantum Computers
References and Further Reading
I. Sivarajah, D. S. Goodman, J. E. Wells, F. A. Narducci, W. W. Smith; Off-resonance energy absorption in a linear Paul trap due to mass selective resonant quenching. Rev. Sci. Instrum. 1 November 2013; 84 (11): 113101. https://doi.org/10.1063/1.4825352
Sivarajah, I (25 May 2023) Mass Spectrometry: A Key Tool in the Fight Against Food Fraud. AZO-OPTICS. Available Online at: https://www.azooptics.com/Article.aspx?ArticleID=2436
Rodríguez, D. A quantum sensor for high-performance mass spectrometry. Appl. Phys. B 107, 1031–1042 (2012). https://doi.org/10.1007/s00340-011-4824-5
Okumura, T., Attri, P., Kamataki, K. et al. Detection of NO3− introduced in plasma-irradiated dry lettuce seeds using liquid chromatography-electrospray ionization quantum mass spectrometry (LC-ESI QMS). Sci Rep 12, 12525 (2022). https://doi.org/10.1038/s41598-022-16641-1
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.