This article primarily explores the uses of atomic excitation and deexcitation in various fields of science and technology.

Image Credit: Sergey Nivens/Shutterstock.com
The planetary atomic model of Niels Bohr (1915) was the first stable model of the atom which gave the concept of electrons revolving around the nucleus in fixed orbits. Erwin Schrödinger’s quantum mechanical atomic model (1926) states electrons revolve around the nucleus in shells, orbits, orbitals, and spinning orientations with discrete quantum states.
Hence, the energy of the electrons is quantized. Atomic excitation means an electron in an atom can be moved to a higher energy orbit when the atom absorbs an amount of energy that exactly equals the energy difference between the orbits. Similarly, atomic de-excitation means, an electron in an atom can be moved to a lower energy orbit when the atom emits an amount of energy that exactly equals the energy difference between the orbits.
Methods of Excitation
Most commonly, atomic excitations are triggered by the absorption of photons with discrete energy that exactly equals the energy gap between two orbits. Many larger atoms are excited using electron- or ion-beams in which fast-moving electrons and ions collide with the electrons in the atom and transfer their kinetic energy through perfect elastic collision. Moreover, some materials such as semiconductors have a very small energy gap, which can be excited simply by thermal excitation. Thermal excitation is a process in which vibrations of crystal lattice provide sufficient energy to transit a ground-state electron to a higher energy state.
When an atom is excited from an already excited state to a higher energy excited state with the absorption of a photon is called excited state absorption (ESA). Most electronically excited states of atoms have lifetimes of a few nanoseconds. Currently, ultrafast femtosecond pulse lasers are used to keep the atoms in the excited state continuously for a long time.
The De-Excitation Process
When an excited electron drops back to the ground state, it radiates its discrete energy in the form of photon, heat, or characteristic electromagnetic (EM) waves. The emitted photon is different from the absorbed photon because there is a loss of energy during the process due to intrinsic molecular vibrations.
Furthermore, the de-excitation process of electrons from the excited state to the ground state can occur in a single step or multiple intermediate steps. In a chemical mixture of two types of molecules, if the excitation state of the first molecule is relatively closer to the ground state of the second molecule than its own ground state, then the excited electron may transfer from the first molecule to the second molecule by radiating photons of longer wavelengths (i.e. lower energy). This is known as fluorescence. Similarly, the spin flipped triplet state de-excitation process is called phosphorescence, which radiates photons of longer wavelength than the fluorescence process.
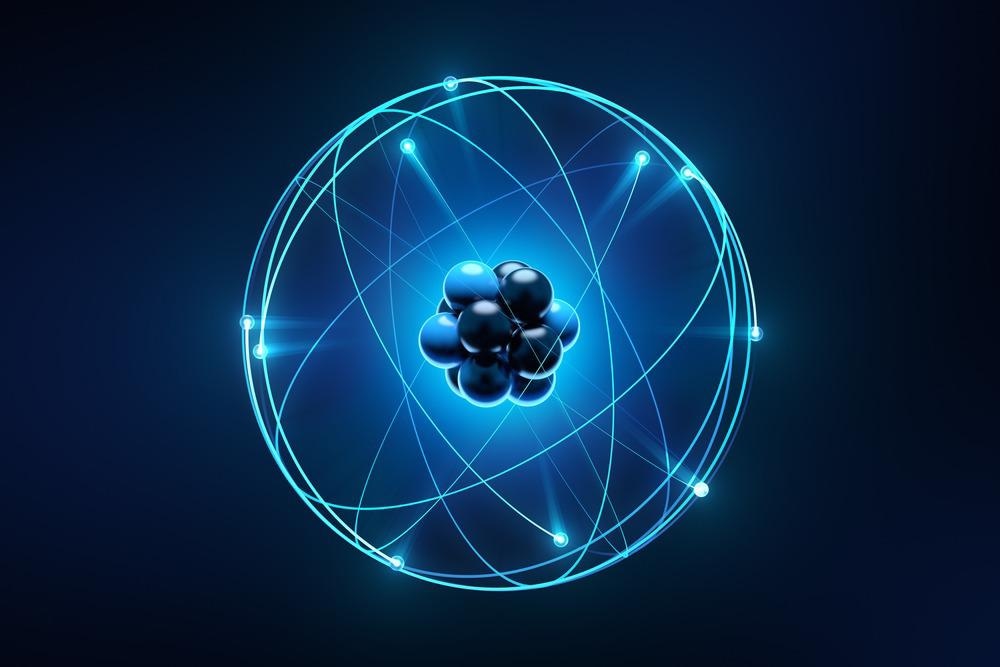
Image Credit: Igor Batrakov/Shutterstock.com
Calculation of Excited States
The excited states of an atom can be quantified using the following methods: coupled-cluster, configuration interaction, Møller-Plesset perturbation theory, flash photolysis, multi-configurational self-consistent field, and time-dependent density functional theory.
Applications of Atomic Excitation and De-Excitation
Fluorescence and phosphorescence methods explained above are used in many biomedical applications such as studying the structure of biomolecules, tracking drug delivery, bio-imaging, and bio-sensing. Additionally, they are used in dyes, paints, and some detergent residues to make the colors of clothes seem brighter in sunlight by converting UV light into visible light. They are also used for brighter X-ray images, neon lights, and gas-discharge tubes. Phosphorescence is used in luminous dials of watches, clocks, and remote-control switches, while thermoluminescence is used for dating ceramic antiquities.
Nanocrystals are a new class of fluorescent materials with a size less than 100 nm. The smallest of them are called quantum dots, which have a size of 2-6 nm. They are more stable, brighter than organic dyes, and have a longer life span.
Lasers are driven by the mechanism of population inversion of electrons. All electrons of the atom of a laser are excited to one state followed by spontaneous de-excitation to a single lower state. This produces coherent photons of a single wavelength which are further amplified using resonating interferences. Lasers are used in various applications such as surgery, machining, welding, weapon guidance, reading compact disks (CDs), producing holograms, and many more.
References and Further Reading
Applications of Atomic Excitations and De-Excitations | Physics. Applications of Atomic Excitations and De-Excitations. https://courses.lumenlearning.com/physics/chapter/30-5-applications-of-atomic-excitations-and-de-excitations/
Development of the Atomic Theory. (n.d.). Development of the Atomic Theory. http://www.abcte.org/files/previews/chemistry/s1_p6.html#:%7E:text=In%201926%20Erwin%20Schr%C3%B6dinger%2C%20an,mechanical%20model%20of%20the%20atom.
Excitation & De-excitation. (n.d.). Excitation & De-Excitation. https://people.wou.edu/%7Ecourtna/ch371/lecture/solar/part2/sld006.htm
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.