Hydrogen may be the Universe’s most common and simplest element, but it is important not to underestimate its importance to the physics of the very large and the infinitesimally small.
Image Credit: Corona Borealis Studio/Shutterstock.com
New research suggests a new model for hydrogen-based upon a fusion of quantum physics and computational science.
This fascination with hydrogen for science isn’t new, and it’s hardly surprising as this element is woven through all of physics, just as it threads through the fabric of the Universe itself.
One proton, orbited by one electron. That’s hydrogen in its most basic form. The Universe’s most common element. Atomic number 1. Sat at the top of the periodic table.
Non-scientists may initially struggle to understand why physicists get so excited about hydrogen. But, any astronomer will quickly correct this.
Stars and Simplicity
For life here on Earth, energy from the sun is everything, and the sun could not produce energy if not for hydrogen.
Every star during its lifetime, which astronomers call the main sequence, is burning hydrogen in its core; the sun is essentially a giant burning ball of hydrogen and some helium.
When the hydrogen in the center of our star is exhausted in around 5 billion years, it will no longer be able to protect itself against gravitational collapse. As its core falls in on itself, the energy released will cause the sun’s outer layers to expand.
This expansion will reach out to the orbit of Mars, meaning the sun, now a red giant star, will consume the inner rocky worlds of the solar system, including Earth.
By the time the pressure in the core is enough to facilitate the burning of helium into heavier elements like carbon, Earth and her sister worlds, Mercury, Venus, and Mars, will be destroyed.
This means that hydrogen in the sun is almost analogous to sand in a cosmic hourglass; when it's gone, our time is up.
Hydrogen in the Early Universe
In the earliest moments of the Universe, mere picoseconds after that initial burst of expansion that we call the Big Bang, the Universe was too hot and dense for any element to exist.
At around 0.000006 seconds the first particles, neutrinos, quarks, and electrons formed. After around three minutes the Universe had cooled enough to reach 10⁹K.
This is known as the era of nucleosynthesis, when the first hydrogen atoms formed, followed by helium atoms. The proportions of these elements in the early Universe were hydrogen at 75 percent, helium at 25 percent, with just a trace of heavier elements; mostly lithium.
More About Our Universe: Direct Detection fo Dark Energy - An Interview With Dr. Sunny Vagnozzi
One of the most impressive aspects of our theory of the Big Bang is the fact that it has been able to precisely predict the relative abundances of these early elements.
These primordial elements went on to form the first generation of stars, which would in turn play host to the nuclear processes that would go on to create the remaining elements forming the rest of the periodic table.
Hydrogen is not just vital at these tremendous cosmic scales, however. It is also pretty important when considering theories of the very small.
Hydrogen as a Simple Quantum Mechanical Model
The idea of quantum physics being simple is probably anathema to most physicists and non-physicists alike. However, hydrogen and its relatively simple composition are vital to helping demonstrate the rules of quantum physics.
The name “quantum” comes from the Latin word “quanta” meaning “how much”, which was adopted by Max Planck when he was first developing the earliest theories of quantum mechanics.
Albert Einstein had previously discovered something called the photoelectric effect, a phenomenon in which light hits metal and causes the release of electrons. The theory would earn Einstein the Nobel in 1921, and is probably commonly encountered by most people when they walk through an automatic door; the opening mechanism of most of these doors is built upon the photoelectric effect.
Here emerges the part of the photoelectric effect that leads to the discuss of quantum physics and hydrogen. What Einstein found was that the intensity of light, the number of photons, shone on a strip of metal, did not influence the release of electrons.
You could shine long-wavelength, low-frequency light on a sheet of metal all day and it would not release a single electron. Switch that out for a high-frequency, short-wavelength light and in seconds electrons would be flying everywhere.
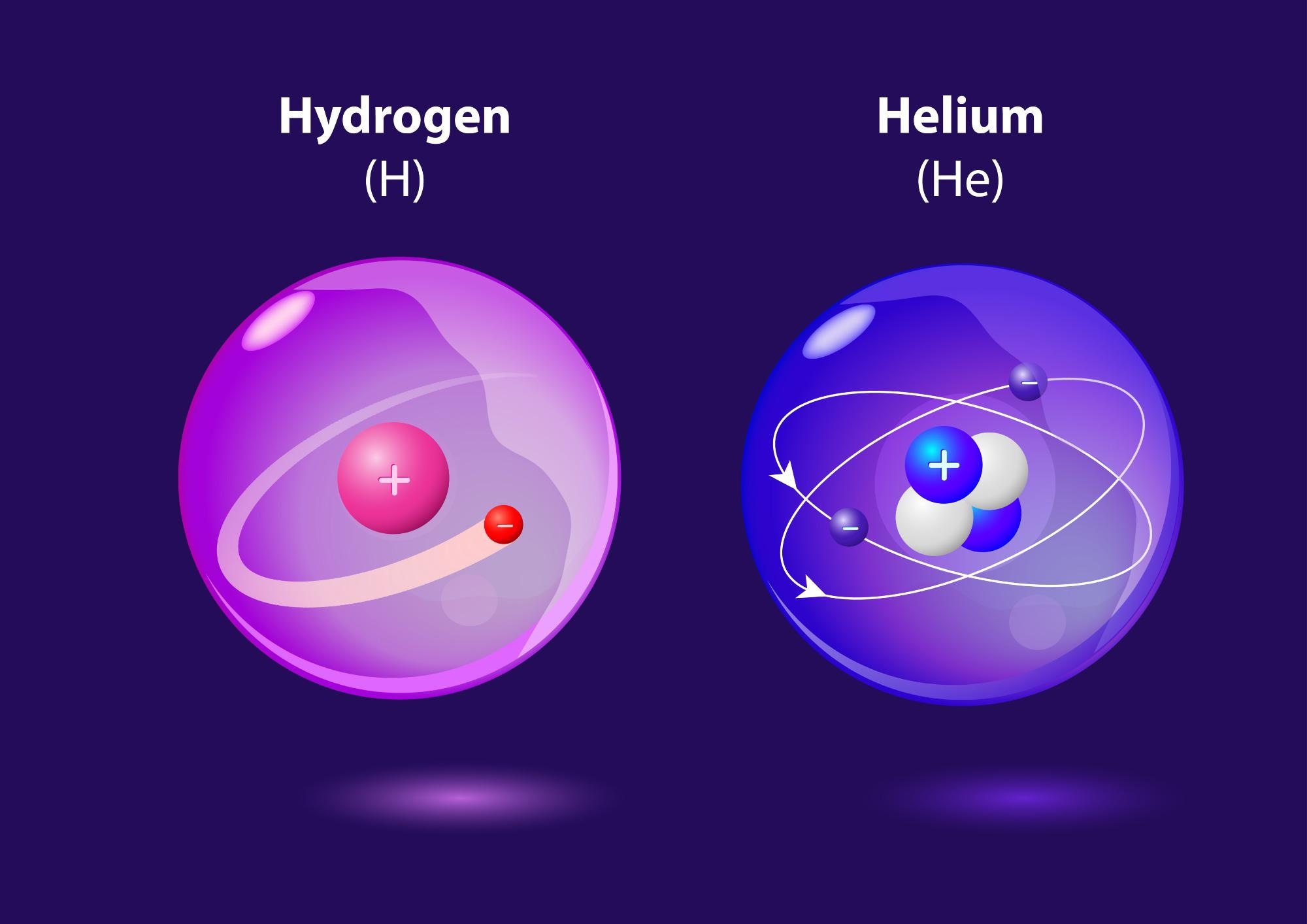
Image Credit: Designua/Shutterstock.com
The reason for this is that photons come as “packets” of energy that could take certain values intrinsically tied to frequency and wavelength. Only one of these packets or “quanta” with enough energy can shake loose an electron.
The best way of considering this is looking at what happens to electrons around atoms. Because multiple electrons can have a screening effect that makes the situation more complicated, the easiest way to do this is by looking at an element that consists of one electron around one proton: hydrogen.
An electron around a proton has a ground state energy of -54.4 electron volts (eV); to liberate that electron — to set it completely free from the proton — you have to get it to absorb a photon of 54.4 eV or more.
Two photons of 27.2 eV will not be enough, nor will photons of 53 eV or 1.4 eV. Only a photon with 54.4 eV or more will liberate that electron.
However, ground state and completely free are not the only energy states for electrons. When around an atomic nucleus, an electron can go through several excited states or electron shells.
For hydrogen, the first excited state can be occupied by an electron with -13.6 eV, the second -6.04 eV, and so on.
So, if one imagines an electron at the ground state in a hydrogen atom absorbing a photon of 40.8 eV, it will “step up” to that first excited state. It won’t stay excited for too long; eventually — and unpredictably — it will emit a photon of 40.8 eV and drop back down to the ground state.
An analogy is that electrons in an atom are like a ladder. Your foot can rest at any rung for a time, just like an electron can sit at any energy state. But, neither your foot nor the electron can rest between rungs or energy levels respectively.
A Hydrogen Thread Linking Science’s Future to Its Past
The dawn of quantum mechanics was at the start of the 20th century, but it is the importance and applicability of hydrogen that is still inspiring research today. Recently it inspired Jeevake Attapatu’s paper, which also acts as this doctoral thesis.
Attapatue takes a thoroughly modern approach to modeling the behavior of hydrogen, using machine learning to understand its conductive properties and its temperature.
Using methods that wouldn’t be available to their peers, modern scientists are still unpicking the mysteries of this allegedly simple element, creating a thread from the future to the past of science.
References and Further Reading:
Williams. M., (2016). “Will Earth survive when the sun becomes a red giant?” Universe Today, [https://phys.org/news/2016-05-earth-survive-sun-red-giant.html#:~:text=In%205.4%20billion%20years%20from,collapses%20under%20its%20own%20weight.]
Gary, D. (n/a). Cosmology and the Beginning of Time, [https://web.njit.edu/~gary/202/Lecture26.html]
Attapatu. J., (2021). ‘Modeling the quantum behavior of hydrogen using density functional theory, quantum Monte Carlo, and machine learning,’ Washington State University, ProQuest Dissertations Publishing. 28414802, [https://www.proquest.com/openview/318b211b6f0364b0d245c54f2de9d3fd/1?pq-origsite=gscholar&cbl=18750&diss=y]
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.