The Bohr Model is a representation of the atom where negatively-charged electrons orbit around a positively-charged nucleus in the same way that the planets of the solar system orbit the sun. In the latter, the force holding the planets and the sun together is a gravitational force; whereas in the Bohr Model of the Atom, the electrons and nucleus are held together by an electrical force.
Brief History of Modern Models of the Atom
1904 - JJ Thomson’s Plum Pudding Model
JJ Thompson’s plum pudding model of the atom is best explained by imagining a plum pudding (or a blueberry muffin if you prefer): the plums in the pudding play the role of negatively charged electrons immersed in a dispersed positively charged pudding. This helped the model to achieve its primary purpose of explaining why most atoms were neutral.
1911 - Rutherford’s Model
Ernest Rutherford arranged for an experiment to take place in 1909 by scientist Hans Geiger and his student Ernest Marsden. The experiment, commonly known as the gold-foil experiment, involved sending a beam of alpha particles through a very thin gold foil.
Under JJ Thompson’s model all the particles were expected to pass straight through the foil; in reality it was found that some of the particles emerged at different angles and some even bounced straight back. These results were evidence against JJ Thompson’s Plum Pudding Model and prompted Rutherford in 1911 to develop his own model of the atom with a positively charged mass at the center of the atom. Thus he invented the concept of the atomic nucleus. This was in sharp contrast to Thompson’s previous model in which the positive charge was dispersed throughout the atom.
1913 - Bohr’s Model
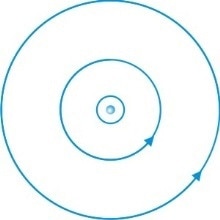
Niels Bohr developed Rutherford’s earlier atomic model by proposing that electrons travel in orbits around the nucleus. His model allowed the outer orbits to hold more electrons than the inner orbits. He also suggested that the number of electrons in the outer orbits determined the chemical properties of the atom.
The electrons in Bohr’s model were not static within their orbits. Bohr proposed that electrons may jump from one orbit to another and suggested that this could be the mechanism behind the emission of radiation. This theory was later developed into quantum mechanics.
The Need for Bohr’s Atomic Model
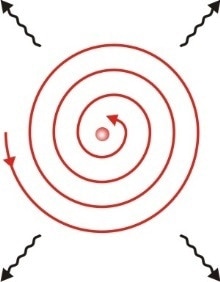
There was a critical problem with Rutherford’s earlier model of the atom: it was not very stable. This was because, according to the rules of physics, electrons orbiting the nucleus would give off energy in the form of photons. This would cause the electrons to spiral into the nucleus and thus the atom could not exist.
What Bohr Proposed
Bohr circumvented the problem with Rutherford’s Atomic Model by proposing that electrons “jump” between orbits when they gain or lose energy. Thus, they do not exist in a state in-between the orbits.
The main points of Bohr’s atomic theory are:-
- Negatively charged electrons orbit a positively charged nucleus in orbits with set energy levels.
- The further away the energy level is from the nucleus, the higher the energy it has. It is thus given a higher number.
- The energy levels hold different numbers of electrons. The smaller the energy level, the lower the number of electrons it holds, e.g. level 1 holds up to 2 electrons, level 2 holds up to 8 electrons and so forth.
- When an electron moves from one orbit to another the atom absorbs or emits radiation.
Problems with Bohr’s Atomic Model
- Bohr’s model does not work very well for complex atoms; for example it does not work very well for atoms with more than one electron in their outer shell.
- The model gives no reason why electrons are solely confined to specific orbits.
- The model does not explain why only a certain number of electrons fit in each orbit; for example why can only 2 electrons fit in the first shell?
- It does not fit with the later developed quantum mechanical principle – Heisenberg’s Uncertainty Principle – because Bohr’s model asserts that both an electron’s radius and orbit can be simultaneously known.
Alterations to Bohr’s Model
In 1916 Arnold Sommerfeld proposed an alteration to Bohr’s model. He suggested that electrons travelled in elliptical orbits. (Incidentally, this is analogous to when Kepler, many centuries earlier, suggested that the planets had elliptical orbits rather than perfect circular ones!)
Sources and Further Reading
This article was updated on the 18th September, 2018.